BIOENGINEERING PLATFORM
Description
The Bioengineering Platform provides a range of skills and tools to support the development and testing of medical devices.
The platform enables the treatment and characterisation of biomaterials, the numerical simulation of complex physiological systems, and the preclinical validation of medical devices of various risk classes.
The technical-scientific staff offers solid skills in numerical modelling, fluid-structural analysis, medical device design optimization, and the conduct of preclinical verifications, based on regulatory requirements and good practice.
The Platform offers a reference point for healthcare organisations, academic centres, and small and medium-sized enterprises in the area, stimulating the production of clinical innovation by local excellence and offering growth paths for new technical and organisational skills in the sector.
Expertise and Support
DEVELOPMENT OF ADVANCED THERAPEUTIC SOLUTIONS
Design and Development of Medical Devices
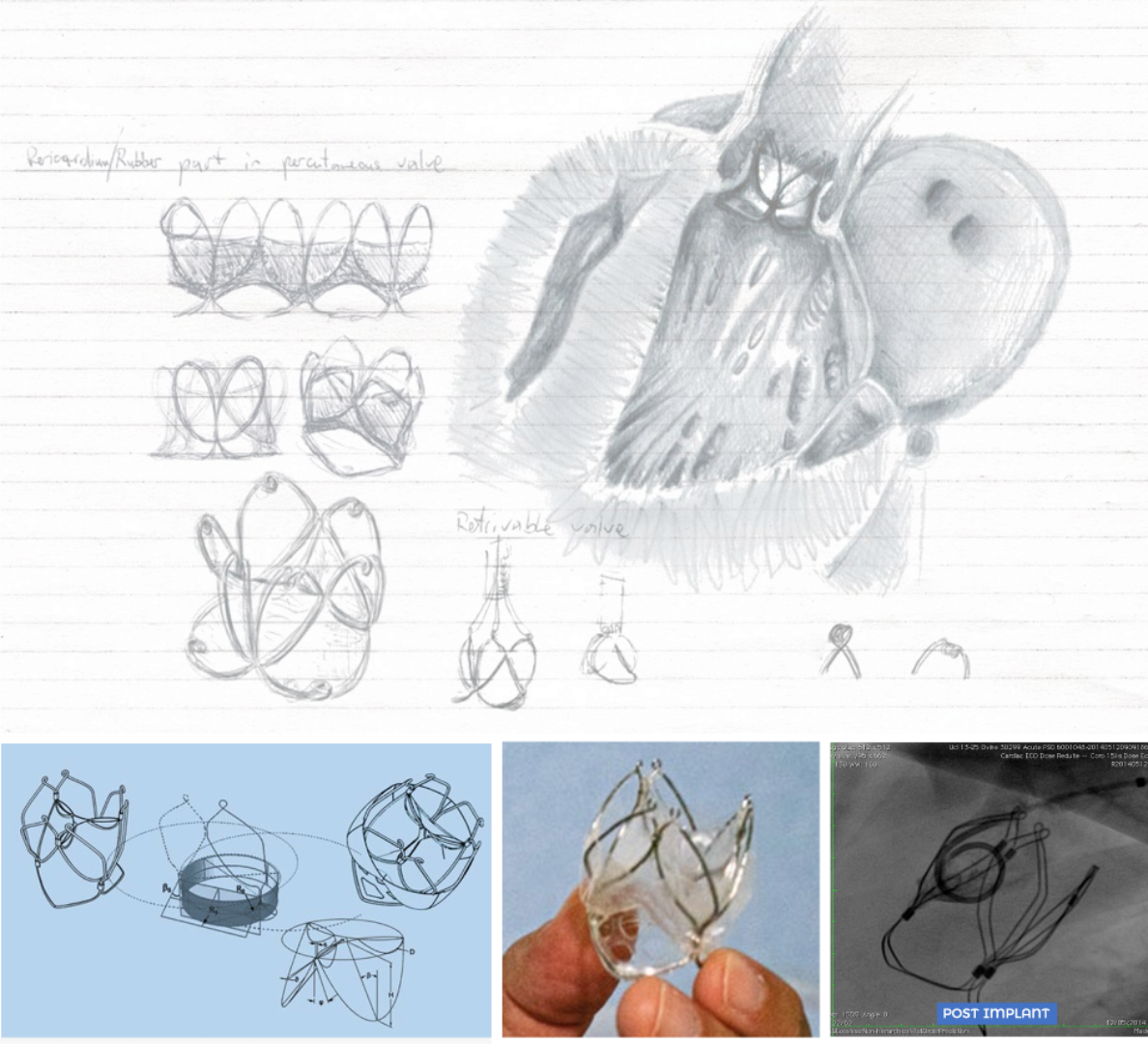
In collaboration with academic partners and the biomedical industry, our researchers contribute to the design and development of innovative medical devices, diagnostic solutions and support tools for therapeutic planning. Some of the devices we have contributed to develop are already helping to define the new generation of cardiovascular therapies, and improve the health conditions and quality of life of patients. These include mitral annuloplasty solutions that provide more physiological responses than existing alternatives, and innovative artificial heart valves.
Examples of medical devices that have been conceived and developed with major contribution from members of our team, and have already found in human application include:
Development of New Diagnostic Techniques and Clinical Support
In collaboration with our academic and clinical partners at UCL/UCLH, at the University of Padua and at the University Hospital Paolo Giaccone of Palermo, we are developing new diagnostic tool to aid diagnosis and risk stratification, assist in the selection of optimum treatments for each specific patient and support the development of new therapeutic strategies by anticipating the safety and efficacy of alternative options.
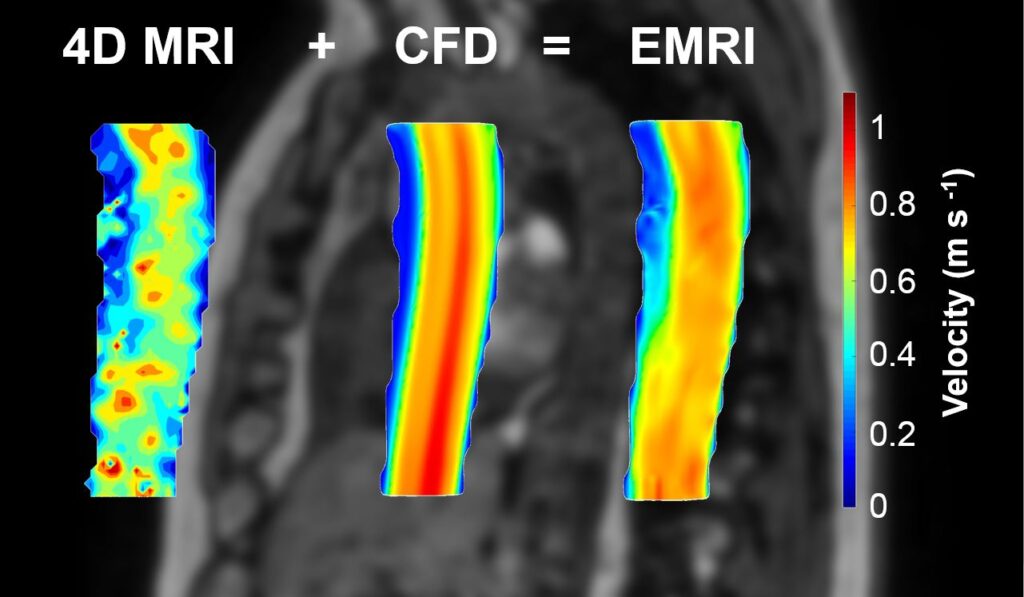
High-resolution time-dependent 3D field of velocity obtained by integrating 4D MR data with computational fluid dynamics (CFD). The enhancement allows to overcome the spatial-resolution limitation of 4DMR. (for more information, see Annio, G., et al., 2021) 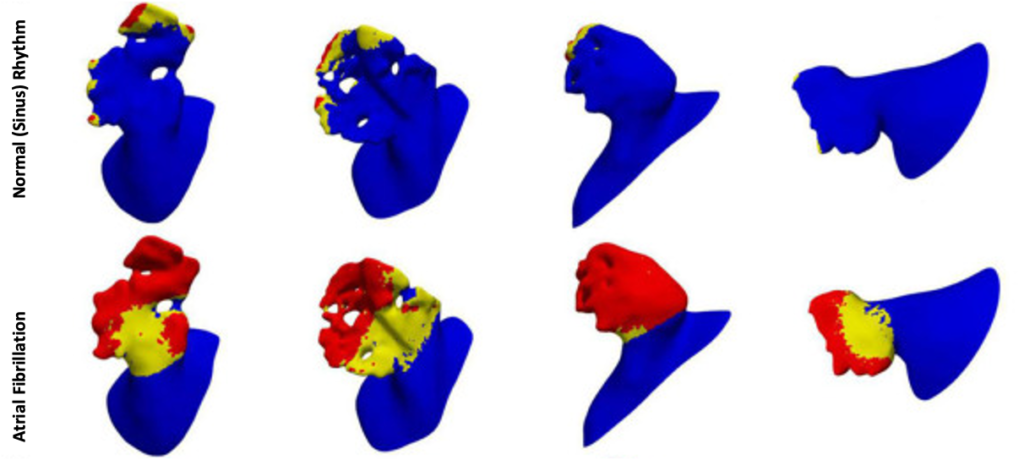
Red and yellow areas indicate the regions where the risk of thrombosis is predicted to be high and medium, respectively, in four patient specific left atrial appendages. Risks are estimated for normal sinus rhythm (top line) and atrial fibrillation conditions (bottom line) – extracted from Musotto, G., et al. Frontiers in Cardiovascular Medicine – Cardiac Rhythmology 2022, 9: 894187. 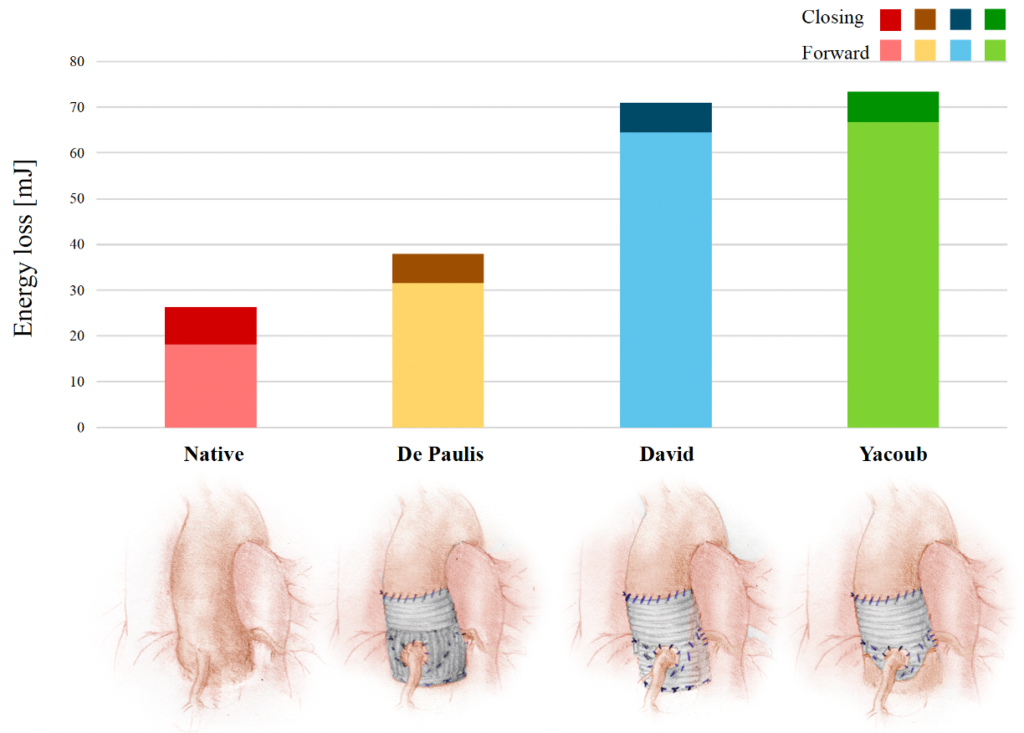
In-vitro quantification of the energy absorved by an aortic valve during the opening/open and closing phases, in healthy conditions and after alternative valve sparing procedures – from Di Leonardo, S., et al. European Journal of Cardio-Thoracic Surgery 63(3): ezad040. Examples of research outcome on diagnostic techniques and support studies for clinical practice include:
- Di Leonardo, S., et al. Hydrodynamic ex vivo analysis of valve-sparing techniques: assessment and comparison. EJCTS 2023, 63(3): ezad040.
- Musotto, G., et al. The Role of Patient-Specific Morphological Features of the Left Atrial Appendage on the Thromboembolic Risk Under Atrial Fibrillation. FCVM 2022, 9: 894187.
- Annio, G., et al. Experimental Validation of Enhanced Magnetic Resonance Imaging (EMRI) Using Particle Image Velocimetry (PIV). Ann Biomed Eng 2021, 49: 3481–3493.
- Salmonsmith, J., et al. Does transcatheter aortic valve alignment matter? Open Heart 2019, 6: e001132.
- Di Micco, L., et al. The neochord mitral valve repair procedure: Numerical simulation of different neochords tensioning protocols. Med Eng Phys 2019, 74:121-128.
NUMERICAL SIMULATION OF COMPLEX PHYSIOLOGICAL SYSTEMS
Numerical analyses support the development and validation of new biomedical devices and the implementation of innovative diagnostic solutions. Our platform allows to conduct advanced simulations of complex systems and interactions between structures and fluid domains.
These analyses include the simulation of structures with complex material behaviour (including hyperelasticity, shape-memory effects and superelasticity), pulsatile physiological flows, as well as the mutual interaction between them.
To date, there is no a unique numerical approach that optimal and efficient for all the phenomena occurring in such systems. The Bioengineering & Medical Devices group and the Bioengineering platform offer the range of expertise and computational tools to cover all main needs of computational bioengineering.
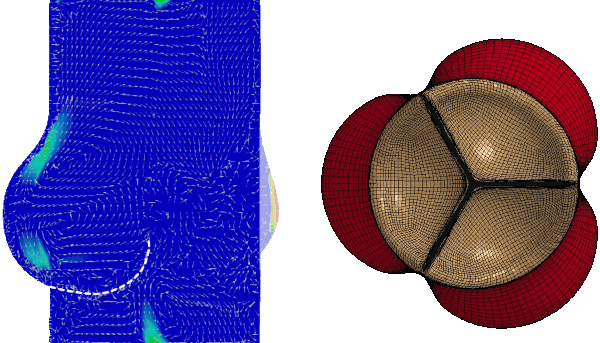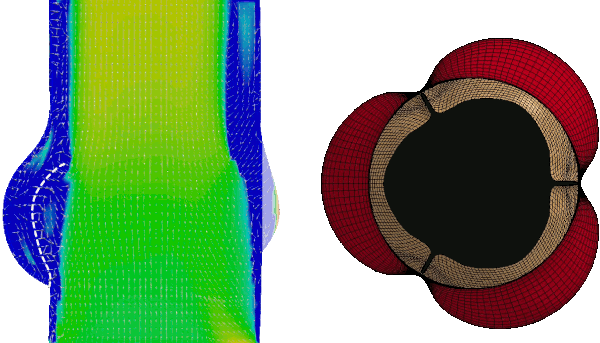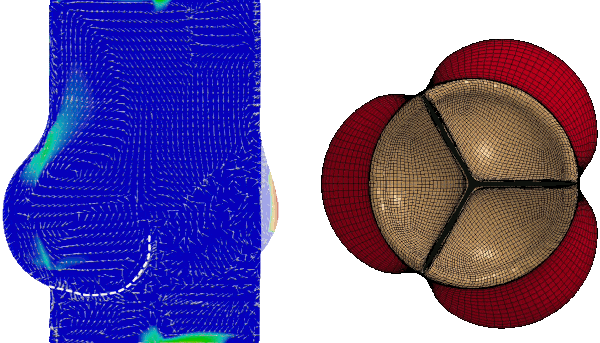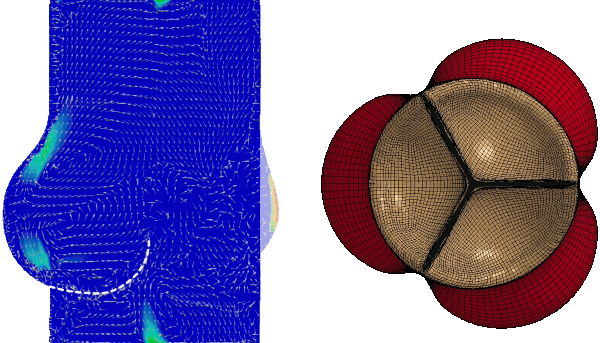
Numerical FSI simulation of the healthy aortic valve function (from Tango et al. Cardiovasc Eng Tech 9, 739–751 (2018). https://doi.org/10.1007/s13239-018-00391-1)
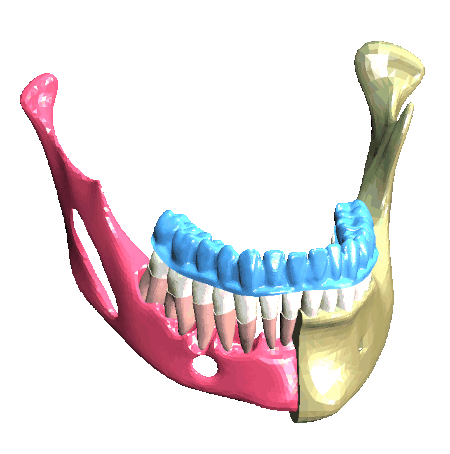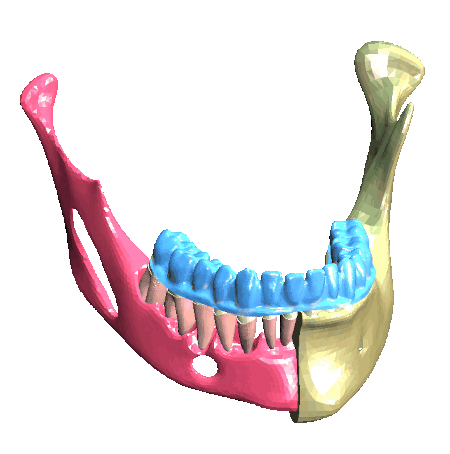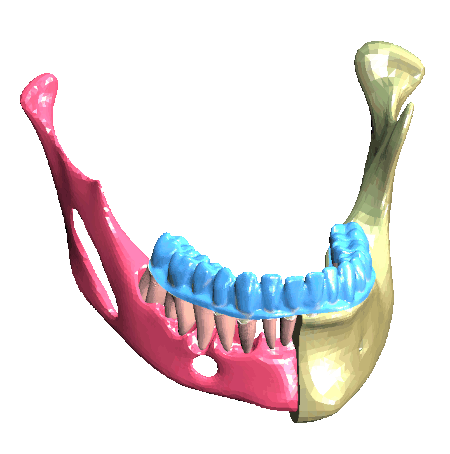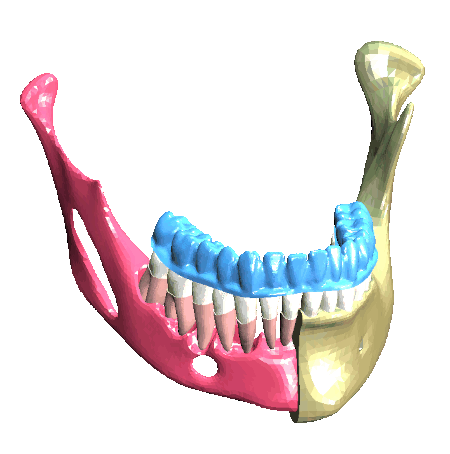
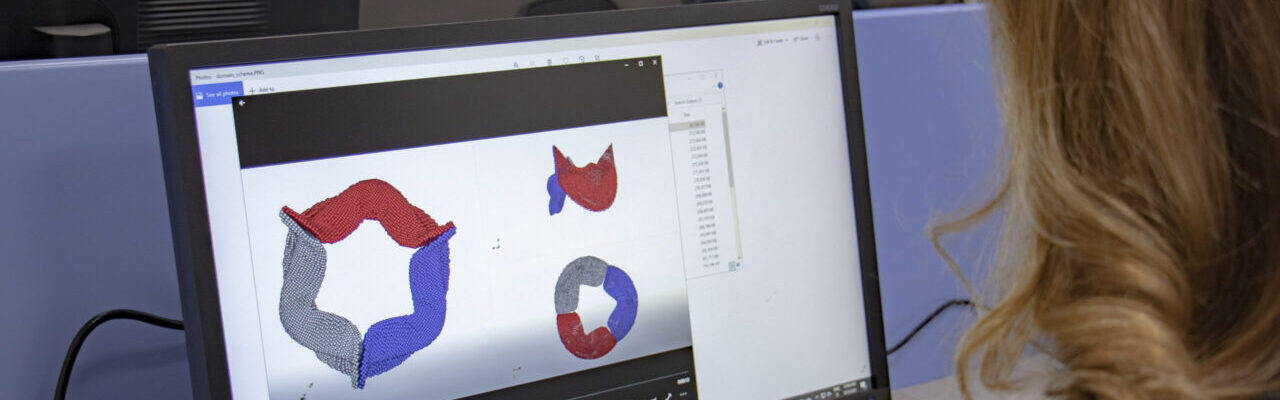
Scheme (on the left) and simulation of the insertion of a clear aligner in the lower dental arch. The model includes the description of the cancellous bone, ligaments, cortical bone, teeth and aligner. (In collaboration with Ferrara School of Orthodontics, University of Ferrara, Italy) 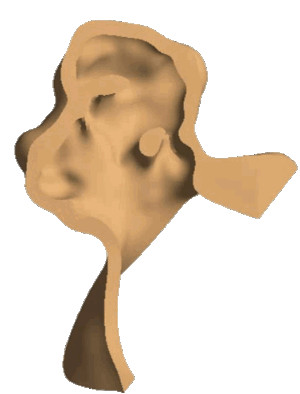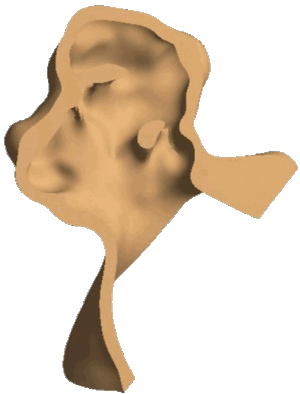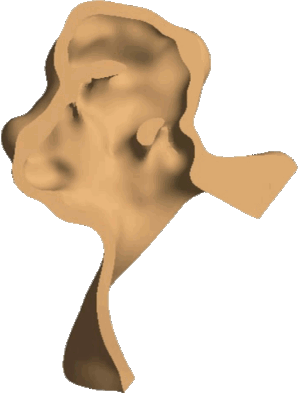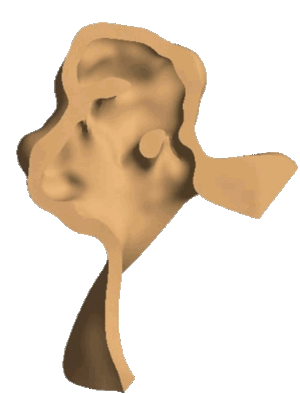
Structural analysis of contracting left atrial appendage. 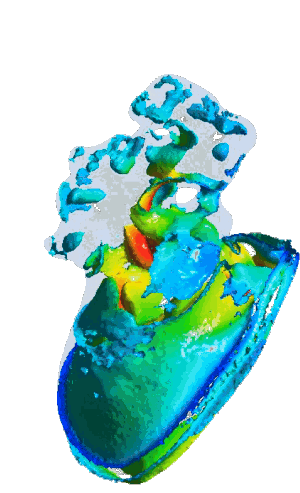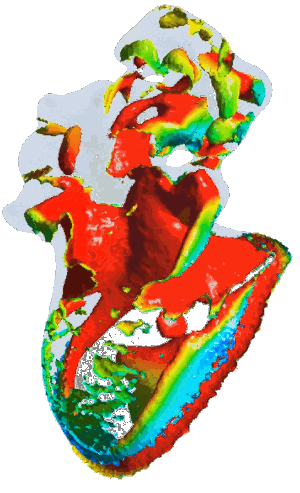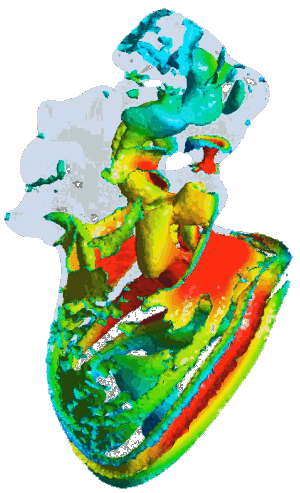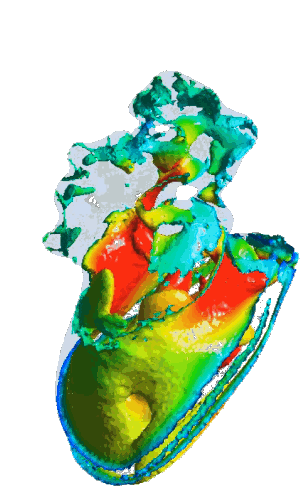
FSI vorticity analysis of a left atrial appendage. 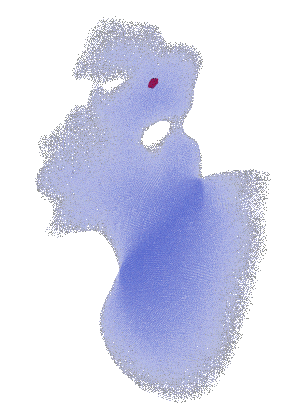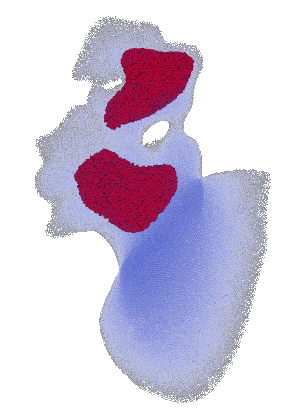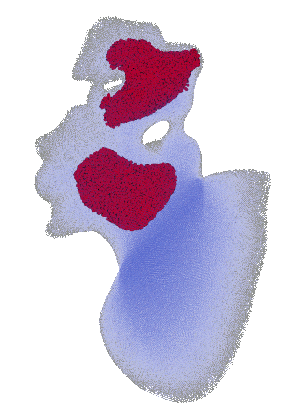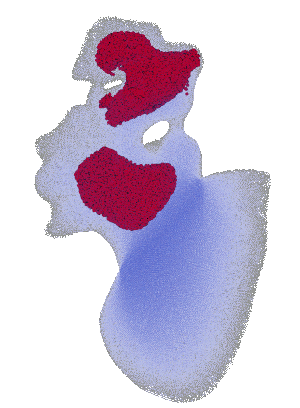
Clot formation in atrial fibrillation predicted with our code. - Monteleone, A., et al. (2023) Modelling of thrombus formation using smoothed particle hydrodynamics method. Plos One 18(2): e0281424.
- Monteleone, A., et al. (2022) Fluid-structure interaction approach with smoothed particle hydrodynamics and particle-spring systems. Comput Methods Appl Mech Eng 392: 114728.
- Vella, D., et al. (2021) Effect of the Alterations in Contractility and Morphology Produced by Atrial Fibrillation on the Thrombosis Potential of the Left Atrial Appendage. Front Bioeng Biotechnol 9: 586041.
- Tango, A.M., et al. (2018) Validation and Extension of a Fluid–Structure Interaction Model of the Healthy Aortic Valve. CVET 9(4): 739-751.
- Tzamtzis, S., et al. (2013) Numerical analysis of the radial force produced by the Medtronic-CoreValve and Edwards-SAPIEN after transcatheter aortic valve implantation (TAVI). Med Eng Phys 35(1): 125-130.
PRECLINICAL IN-VITRO EVALUATION OF CARDIOVASCULAR DEVICES
Haemodynamic Assessment
Prototypes of new cardiovascular implants need to demonstrate their safety and efficacy before being introduced into the clinic. Our platform allows to perform the in vitro tests requested by the international standards, including hydrodynamics, thrombogenic and haemolytic potential.
In particular, we assess the hydrodynamic performance in pulse-duplicator systems, evaluating the efficiency of the device for a range of physiological and pathological conditions.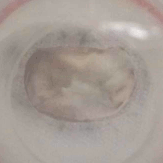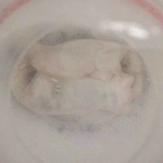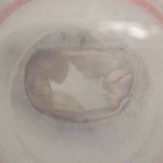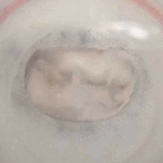
Example of a test on an ex-vivo prolapsing mitral valve. 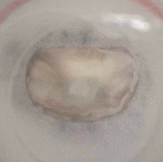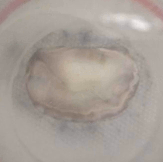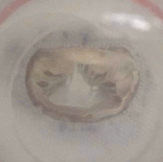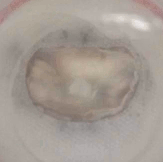
Same valve after procedure to restore healthy operating conditions. Thrombogenic and haemolytic potential is assessed by identifying stagnation regions and region of potential blood damage, commonly by means of particle image velocimetry technique.
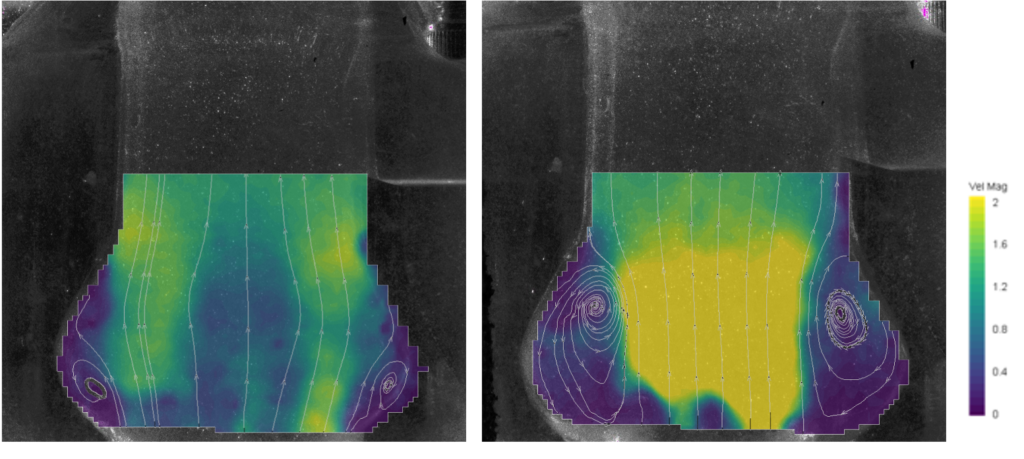
Comparison between the peak systolic flow measured for a mechanical (left) and a biological (right) valve. Durability Assessment
High Cycle durability test on a heart valve prototype manufactured in our platform. We also evaluate the expected lifetime of the devices by means of high-cycle durability testing, cycling them under conservative accelerated physiological conditions for up to 600 million of cycles.
Part of our work is devoted to redefine current international guidance, suggesting enhancement on the practice, that improve the significance of our tests.
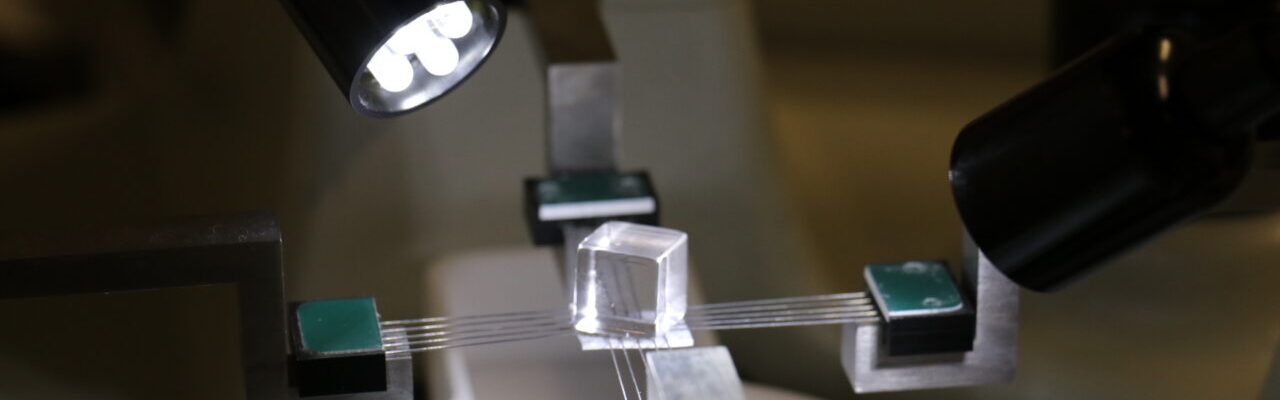
Radial Force Characterisation
Accurate characterisation of new devices such as transcatheter valves, stent, stent graft, is essential for their development. In particular, evaluation of the anchoring force, which depends on the circumferential force that the device exert on the anatomy, is essential in the design and sizing phase. We evaluate the anchoring force by performing radial force tests.

Examples of studies based on our in vitro expertise are:
- Yacoub, M., et al. (2023) Valvulogenesis of a “living”, innervated pulmonary root induced by an acellular scaffold. Commun Biol 2023, 6: 1017.
- Rahmani, B., et al. A Durable Porcine Pericardial Surgical Bioprosthetic Heart Valve: a Proof of Concept. JCTR 2019, 12(4): 331-337.
- Bozkurt, S., et al. Design, Analysis and Testing of a Novel Mitral Valve for Transcatheter Implantation. Ann Biomed Eng 2017, 45(8): 1852-1864.
- Rahmani, B., et al. In-Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (The TRISKELE). Cardiovasc Transl Res 2017, 10(2):104-115.
- Ducci, A., et al. Transcatheter aortic valves produce unphysiological flows which may contribute to thromboembolic events: an in-vitro study. Journal of Biomechanics 2016, 49(16): 4080-4089.
MECHANO-RHEOLOGICAL CHARACTERISATION OF BIOMATERIALS AND BIOFLUIDS
Understanding and characterising the mechanical response of biomaterials is essential for designing and developing safer and more effective clinical solutions.
Our team studies the mechanical and thermo-mechanical behaviour of a wide range of biomaterials and material structures, both natural and synthetic, that are relevant for medical applications and devices. These include hydrogels, soft and hard tissues, polymeric materials, biocompatible metals, cellular structures, and ceramics.
Where applicable, we perform our tests based on regulatory guidelines. Still, in collaboration with our academic partners at the University of Palermo, we are continuously working to redefine current test practice, identifying its limits and proposing new, substantially more accurate and systematic testing approaches.
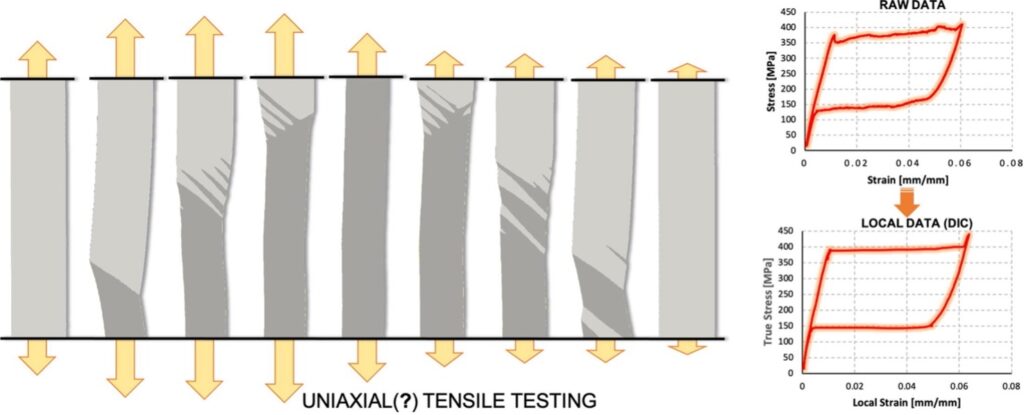
Sequence of deformations in a standard uniaxial tensile test on a nitinol specimen (left – horizontal displacements are amplified of 10 times); and stress–strain curve obtained from standard testing (top right) and with our proposed method (bottom right) – from Materials & Design 2022, 216: 110538 We also enhance the functional response of biological and synthetic materials by designing new metamaterials and introducing genetic engineering concepts.
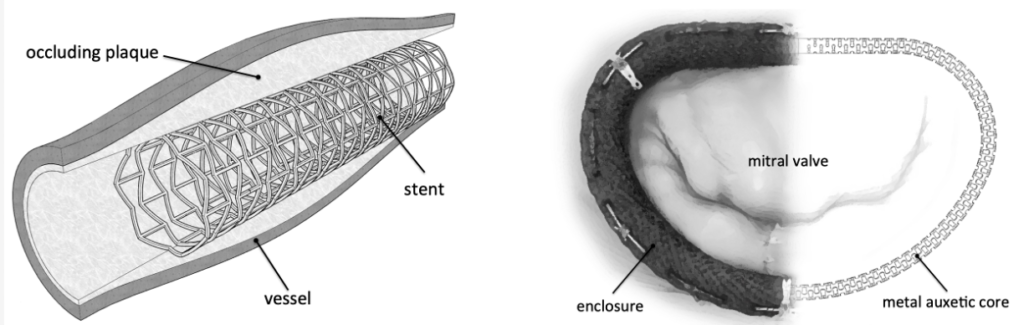
Applications of auxetic (negative Poisson’s ratio) cellular structures in cardiovascular applications, including stents (left) and annuloplasty rings (right) – from Smart Materials and Structures 2013, 22(8): 084008 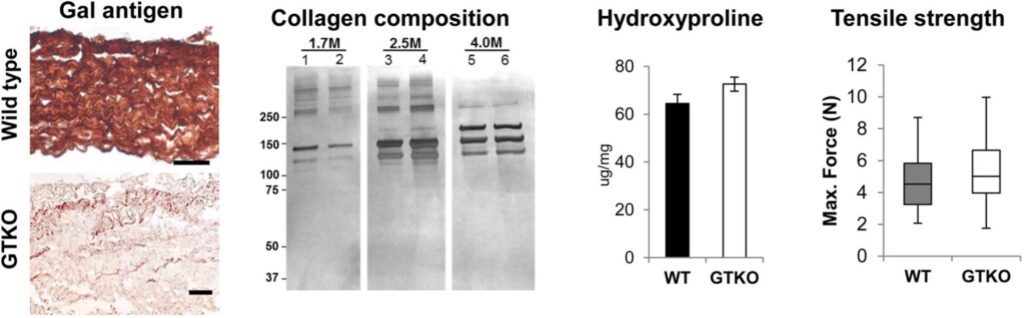
Morphology, composition, hydroxyproline content and tensile strength determined for wild type and Gal-free swine pericardial tissues, a new proposed material for bioprosthetic heart valves – from Acta Biomateralia 2016, 41: 204–209 EXAMPLE OF OUTCOMES AND DISSEMINATION
- Di Leonardo, S., et al. Standard mechanical testing is inadequate for the mechanical characterisation of shape-memory alloys: Source of errors and a new corrective approach. Mater Des 2022, 216: 110538
- Di Leonardo, S., et al. Investigation of the Thermomechanical Response of Cyclically Loaded NiTi Alloys by Means of Temperature Frequency Domain Analyses. Materials 2021, 14: 7866
- McGregor, C., et al. Physical equivalency of wild type and galactose α 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves. Acta Biomater 2016, 41: 204–209
- Karnessis, N. et al. Uniaxial and buckling mechanical response of auxetic cellular tubes. SMS 2013, 22(8): 084008
- Ghanbari, H., et al. The anti-calcification potential of a silsesquioxane nanocomposite polymer under in vitro conditions: Potential material for synthetic leaflet heart valve. Acta Biomater 2010, 6(11): 4249-4260
MANUFACTURING EXPERTISE
Bioengineering research is intrinsically characterised by a high degree of customisation, where systems and devices often need to meet specific needs of a patient or pathological context, demonstrating adequate performance and safety.
Hence our team is often engaged in the definition of novel manufacturing approaches, either aimed at the production of biomedical devices and systems or to develop purposely made new more effective testing equipment.
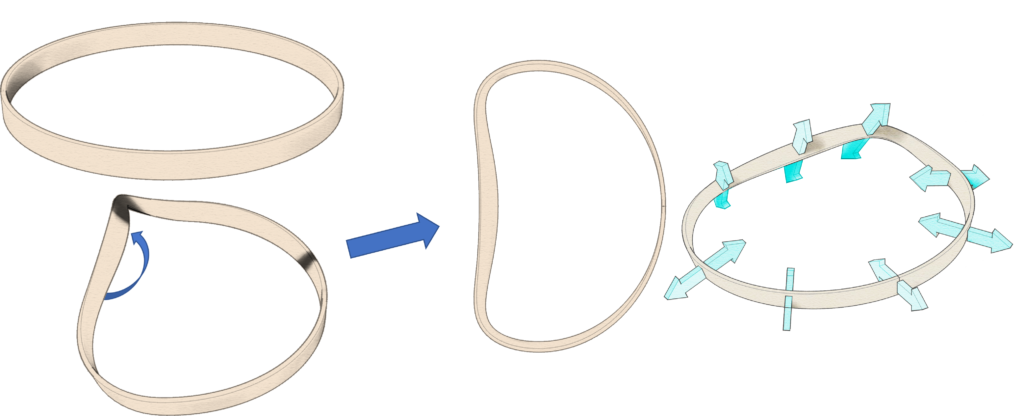
Construction of a novel semi-rigid annuloplasty ring by twisting one portion of a circular band, thus resulting in a correct three-dimensional anatomical shape enforcing optimum physiological dynamics (from Burriesc, G. (2019) Semi-rigid annuloplasty ring and method of manufacturing. WO2019220365). 
Construction steps defined for a new bioprosthetic heart valve currently under preclinical investigation (from Burriesci, G., et al. (2018). Prosthetic heart valve. WO2018011592). Vascular phantoms mimicking human vessels are commonly used to perform in vitro hemodynamic studies for a number of bioengineering applications, such as medical device testing, clinical simulators, and medical imaging research.
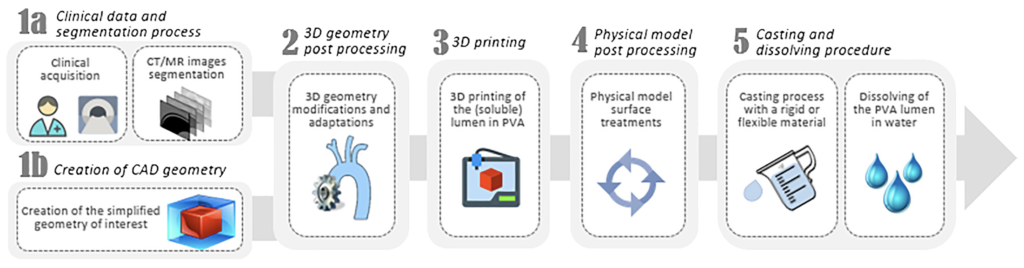
Manufacturing procedure set for the realisation of anatomical phantoms suitable for enhanced in-vitro assessment (from Annio, g., et al. Low-Cost Fabrication of Polyvinyl Alcohol-Based Personalized Vascular Phantoms for In Vitro Hemodynamic Studies: Three Applications. ASME J of Medical Diagnostics. 2020, 3(3): 034501)

Example of manufacturing of a patient-specific calcified aortic root. 
3D printed models of patient-specific left atrial appendages used for in-vitro assessment of blood stasis. - Annio, G., et al. Low cost fabrication of PVA based personalized vascular phantoms for in vitro haemodynamic studies: three applications. ASME J of Medical Diagnostics 2020, 3(3): 034501.
- Provaggi, E., et al. 3D printing assisted finite element analysis for optimising the manufacturing parameters of a lumbar fusion cage. Mater Des 2019, 163: 107540.
- Maneas, E., et al. Anatomically realistic ultrasound phantoms using gel wax with 3D printed moulds. Phys Med Biol 2018, 63(1): 015033(10pp).
- Rahmani, B., et al. Manufacturing and hydrodynamic assessment of a novel aortic valve made of a new nanocomposite polymer. J Biomech 2012, 45(7): 1205-1211.
- Sarkar, S., et al. Manufacture of small calibre quadruple lamina vascular bypass grafts using a novel automated extrusion-phase-inversion method and nanocomposite polymer. J Biomech 2009, 42(6): 722-730.
PROFESSIONAL TRAINING
Our team members have experience in the design, structure and implementation of academic courses, and regularly contribute to the teaching at local and world’s top international universities.
 We provide professional training in the area of design and qualification of medical devices to graduates and technicians working in the biomedical industry.
We provide professional training in the area of design and qualification of medical devices to graduates and technicians working in the biomedical industry.
We are also engage with patients and the public of different age, to ensure that the public is aware of and understands the value and impact of bioengineering, and technologies are fully communicated to potential patients.


Contact:
How to reach us:
Viale delle Scienze, Ed. 18
90128 Palermo, ITALY
Facilities and Equipment
COMPUTATIONAL TOOLS
The platform of Bioengineering is equipped with commercial and in-house numerical codes allowing to perform advanced numerical simulations of complex physiological systems, development/optimisation of new medical devices and generation of in silico models for clinical stratification of the risk related to specific diseases.
These include software for computational fluid dynamics (CFD), for computational structural dynamics (CSD) and for fluid-structure interaction (FSI) modelling.
In particular, the platform is equipped with commercial implicit and explicit solvers and in-house codes for the modelling of ad-hoc applications:
- ANSYS® Mechanical and CFD. This includes CFX which is an implicit solver based on finite volume method (vertex-centered approach); Fluent which is an implicit and explicit solver based on finite volume method with a (cell-centered approach), ANSYS Mechanical for static and transient CSD analysis) and ANSYS System coupling approach for FSI applications;
- LSTC LS-DYNA for CFD (compressible and incompressible fluid flows), finite element analysis (FEA) with explicit solver and FSI with immersed boundary approach;
- ABAQUS FEA for CSD with implicit and explicit solvers;
- MSC.Marc/Mentat for CSD with implicit FEA solver;
- Smoothed particle hydrodynamics (SPH) with an incompressible approach for CFD and FSI modelling.
ANSYS
ANSYS® Mechanical and CFD, produced by ANSYS Inc (Canonsburg, Pennsylvania, USA) allows to describe the mechanical response of complex anatomical structures and the motion of biofluids in the cardiovascular field, guaranteeing accurate and efficient solutions in acceptable calculation times.
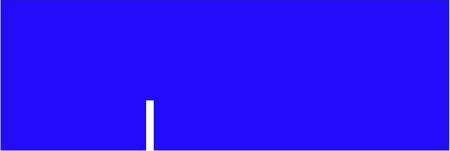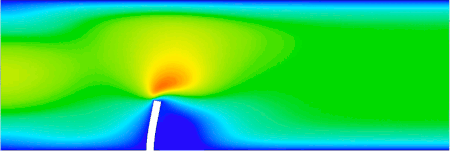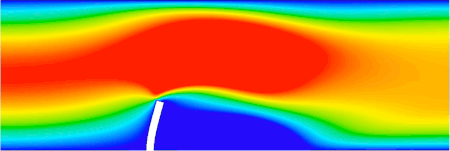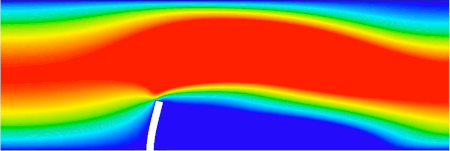
FSI analysis of an elastic beam immersed in a channel flow (ANSYS software, implicit analysis) LS-DYNA
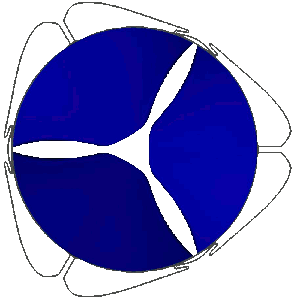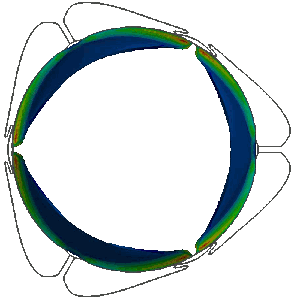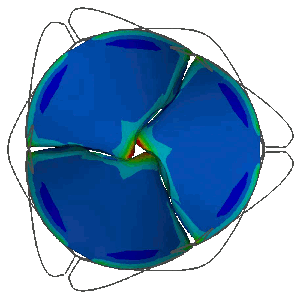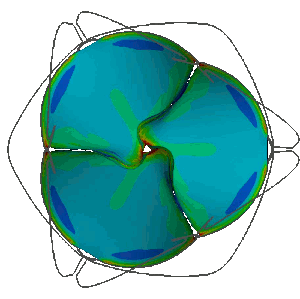
Stress distribution in the leaflets of a prosthetic valve predicted with LS-DYNA. LSTC LS-DYNA, produced by ANSYS Inc, offers unique performance to carry out realistic simulations of FSI phenomena involving the coupling of highly deformable anisotropic non-linear structures with pulsating biofluid flows. This allows the analysis of the mechanical behaviour of heart valves.
ABAQUS
Abaqus FEA offers powerful, comprehensive solutions for numerical simulations of complex physiological systems. The package includes an extensive range of material models such as elastomeric (rubberlike) and hyperelastic (for soft tissue modelling).
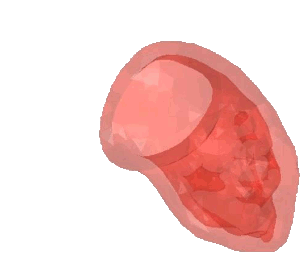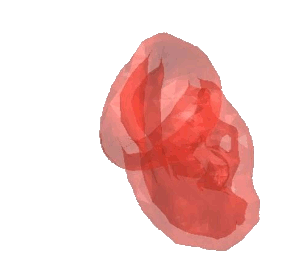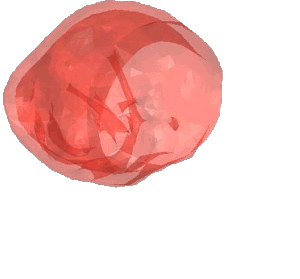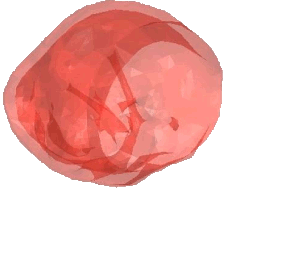
CSD Simulation of the LAA inversion (ABAQUS software, explicit analysis). 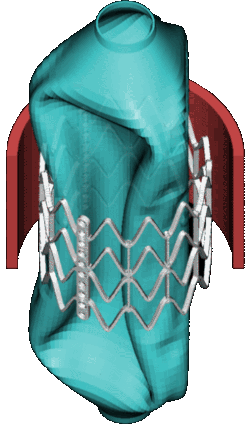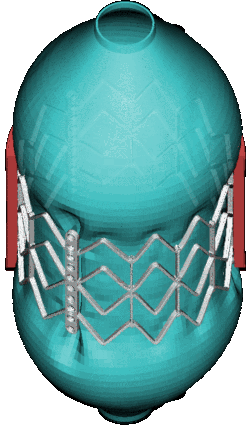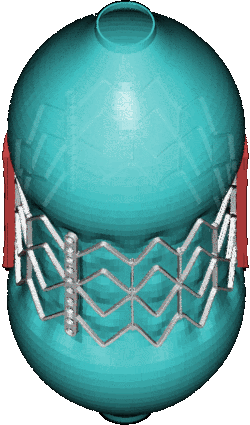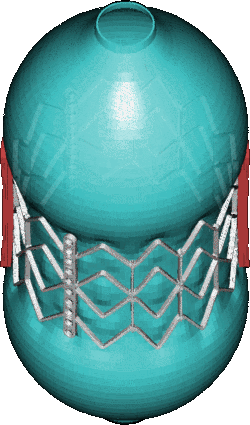
CSD Simulation of the balloon used in transcatheter implant (Marc MENTAT software, implicit analysis). MSC.Marc/Mentat
Marc/Mentat is an implicit non-linear analysis software. It is particularly suitable in the assisted design of cardiovascular devices for transcatheter application, of stents in superelastic/shape memory Nitinol alloys. It allows to simulate structures with highly non-linear behaviour (elastomeric and hyperelastic materials).
Smoothed Particle Hydrodynamics
Smoothed particle hydrodynamics (SPH) is a Lagrangian particle method where the fluid is represented by a finite number of particles. Thanks to the mesh-free feature, SPH conveniently treats multi-phase processes, highly complex geometries and large deformations, efficiently capturing rapidly moving interfaces.
The Bioengineering Computational Unit contributes to develop an open-source SPH code implemented at the Department of Engineering of the University of Palermo, Italy. Due to the versatility of the code, several in-house algorithms were developed for specific bioengineering solutions, for which the classical Eulerian approaches are inadequate.
These includes:
- Fluid-structure interaction (FSI) techniques;
- Thrombus formation method.
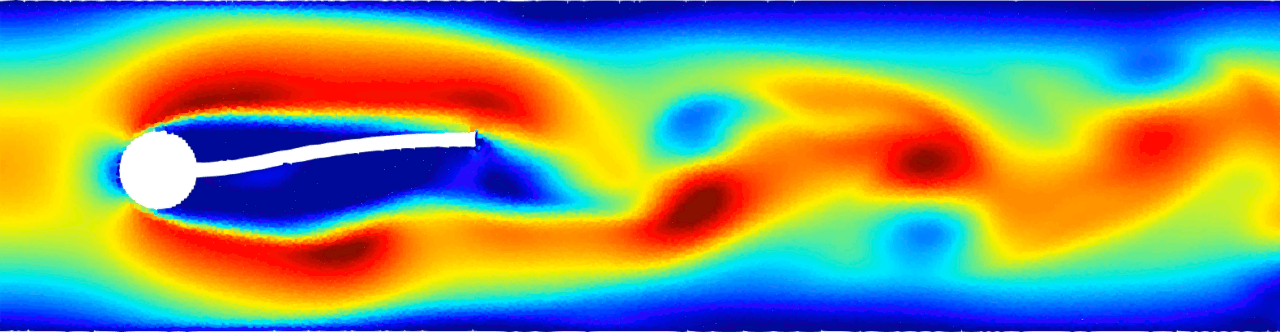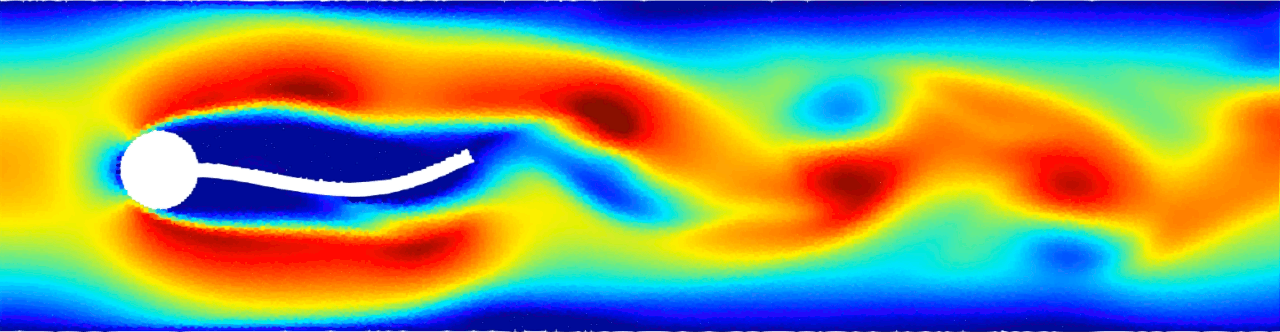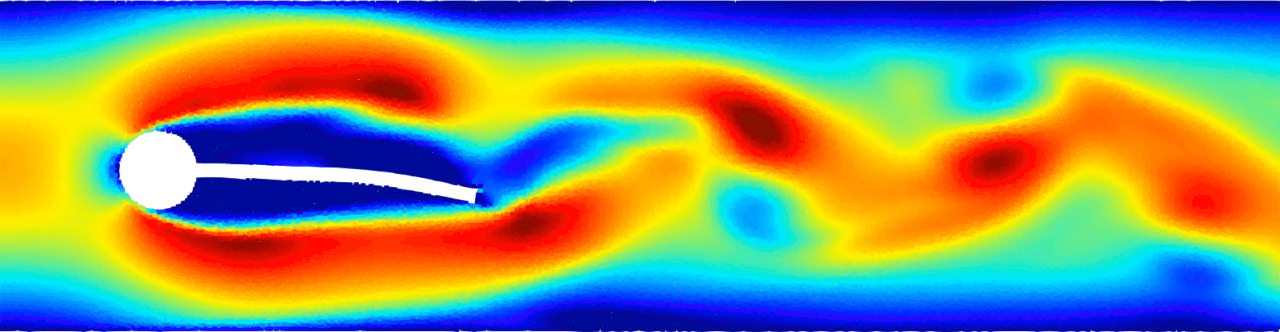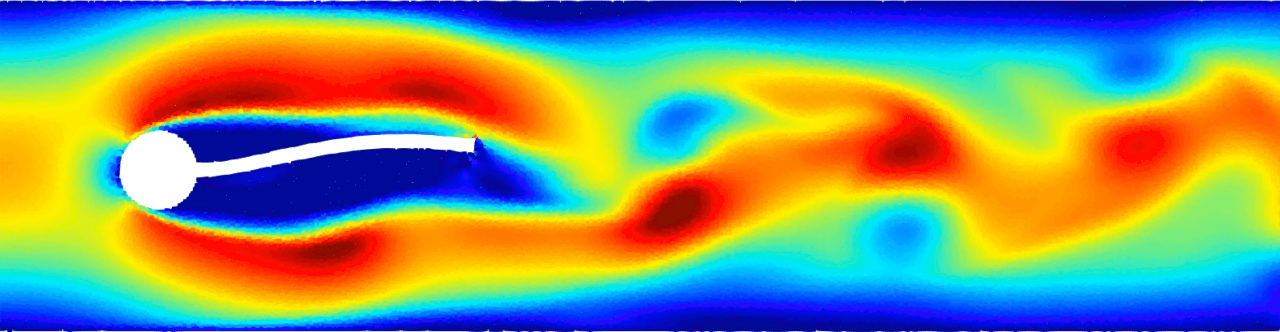
Flow-induced vibration of an elastic beam behind a cylinder. This is a challenging benchmark test case typically used to validate FSI solvers. Von Kármán vortex forms as a consequence of the unsteady separation of flow of a fluid around an immersed body and consequently the beam deforms. For more details see Monteleone, A., et al., 2022. doi.org/10.1016/j.cma.2022.114728 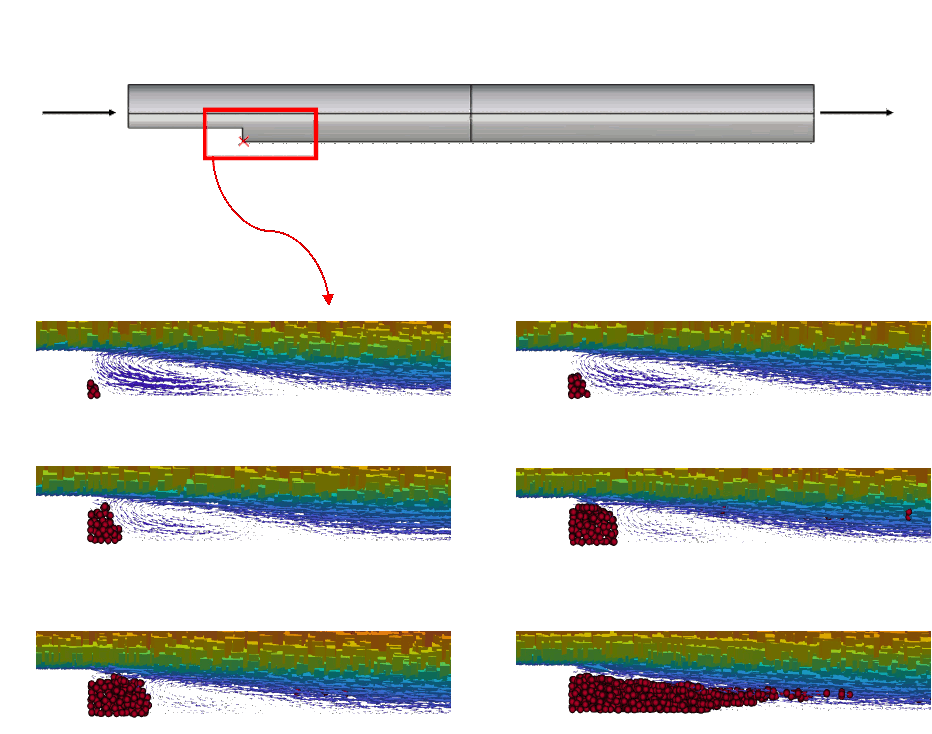

Simulation of the thrombus formation in a backward-facing step. Due to the complexity of the process, commercial numerical codes, based on conventional approaches, do not allow to simulate thrombus formation. For more details see Monteleone, A., et al., 2023. doi.org/10.1371/journal.pone.0281424 IN-VITRO EVALUATION FACILITIES
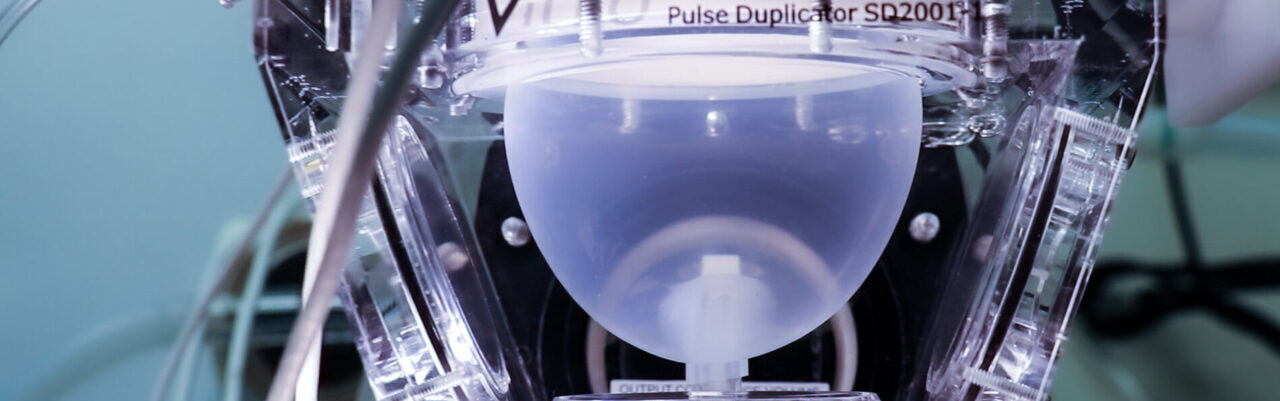
Hydromechanical Cardiovascular Pulse Duplicators
Two ViVitro Superpump System SP7084 (ViVitro, Victoria, BC, Canada) are available in the Bioengineering Platform.
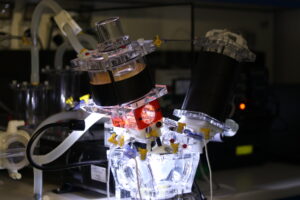 These are pulse duplicator apparati that allow to reproduce physiological heart conditions of of the systemic or pulmonary side. One is set up for aortic hydrodynamic assessment, and the other for mitral valve hydrodynamic assessment.
These are pulse duplicator apparati that allow to reproduce physiological heart conditions of of the systemic or pulmonary side. One is set up for aortic hydrodynamic assessment, and the other for mitral valve hydrodynamic assessment.Both devices are customised to provide enhanced anatomical and physiological description and allow to include ex vivo semilunar and atrio-ventricular valves, mock compliant aortic roots with native mock leaflets for percutaneous implants assessment, patient specific phantoms (e.g. of left atrial appendages) coronary flows, physiological temperature control, continuous viscometrical monitoring and high speed-cameras for analysis of the valve dynamics.
The systems are adapted to provide optimum optical access for coupling with PIV facilities and visualisation/measurement of the main flow parameters associated with blood damage.
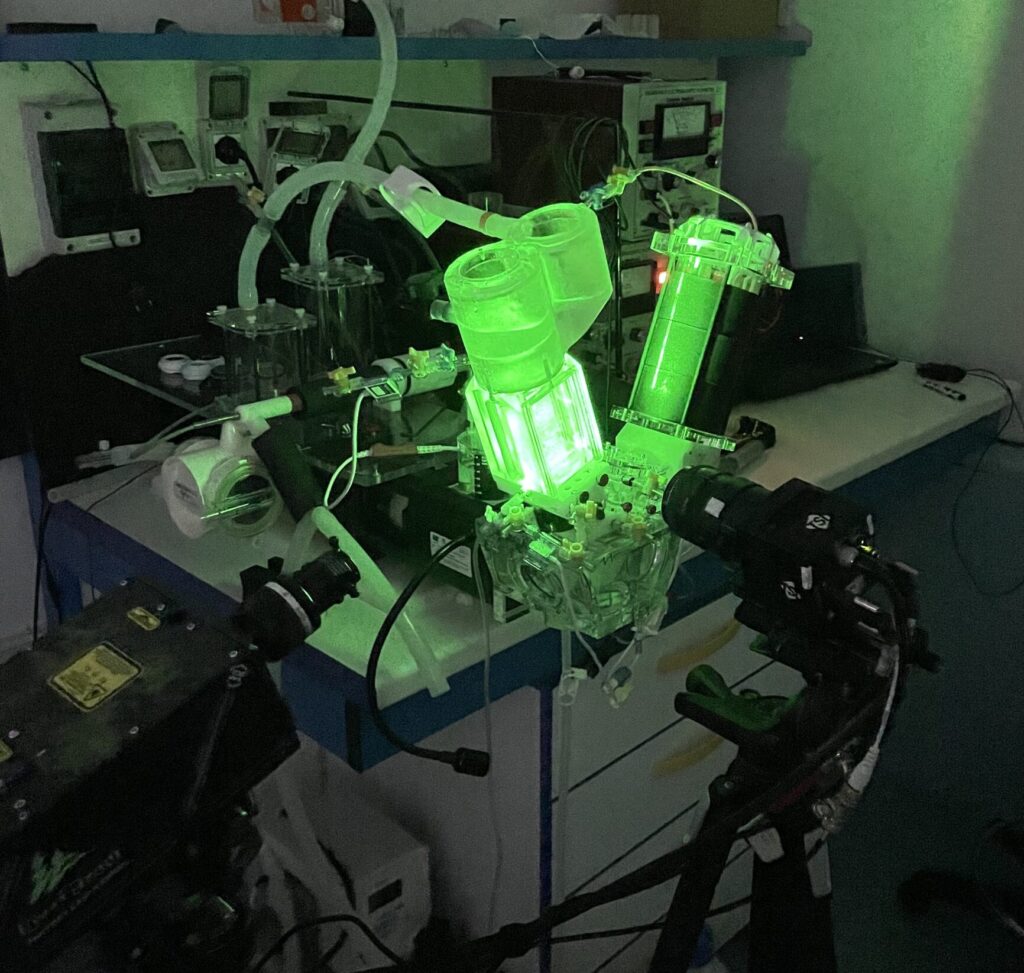
Customised pulse duplicator adapted for PIV analysis. Particle Image velocimetry systems
Three Particle Image velocimetry (PIV) systems are available in the Bioengineering Platform:
- time-resolved 2D PIV system;
- phase-resolved 2D-PIV system;
- phase-resolved Stereo-PIV system.
These can be combined with the pulse duplicator system to assess the thrombogenic and haemolytic potential of heart implants, as recommended by the international standard ISO 5840 on Cardiovascular implants of cardiac valve prostheses.
In particular, the fluid is seeded with neutrally buoyant particles, which are selectively enlightened with a laser. The movement of the brightened particles is recorded by cameras on a sequence of instants, and the analysis of the acquired images though correlation functions allows to estimate the velocity field.
 PIV allows to capture whole field velocity information of a fluid in motion. In parti
PIV allows to capture whole field velocity information of a fluid in motion. In partiOur time-resolved PIV system comprises a high-speed camera and a high repetition rate laser, allowing to measure the temporal development of a flow field.
The phase-resolved PIV systems comprise one (2D) or two (Stereo-PIV) cameras and a double-pulse laser, and are more suitable for the analysis of periodic flows. Stereo-PIV are preferable when the out of plane velocity component is significant, as the combination of the pair of images of each camera allows to reconstruct all three components of velocity in the laser plane.
Ultrasound Imaging

To perform ultrasound imaging, we use a Logiq E portable ultrasound machine from GE Healthcare. This system is capable of performing all essential ultrasound functions, including color doppler for blood flow visualisation, pulsed wave (PW) doppler for local flow velocity measurement, and continuous wave (CW) doppler for high flow velocity measurements. The machine is equipped with a series of probes suitable for various cardio–vascular applications, and includes the TVI (Tissue Velocity Imaging) software for the analysis of tissue movement.
Durability Assessment
 Two VDT-3600i Valve Durability Test Systems (BDC Laboratories CO, USA) are available in the Bioengineering Platform to perform mechanical durability testing and dynamic characterization of cardiac implants, materials and test specimens in general. Each system consists of up to six independent dynamic pressure drives (12 in total), capable of a high frequency oscillatory motion, coupled with sample fixtures, a pressure distribution chamber, a test head and temperature control.
Two VDT-3600i Valve Durability Test Systems (BDC Laboratories CO, USA) are available in the Bioengineering Platform to perform mechanical durability testing and dynamic characterization of cardiac implants, materials and test specimens in general. Each system consists of up to six independent dynamic pressure drives (12 in total), capable of a high frequency oscillatory motion, coupled with sample fixtures, a pressure distribution chamber, a test head and temperature control.For heart valves testing, continual monitoring of the real-time differential pressures in each test stations allows to verify the adherence to ISO 5840 testing recommendations, and to set individual station alarms to halt the system if loading conditions do not meet requirements.
Our system is customised to maximise specimen exchangeability with the pulse duplicator and ease intermediate hydrodynamic assessments with minimum specimen manipulation, and can host compliant mock arteries, TAVI/TAVR devices, stented and stentless flexible leaflets valves and mechanical valves.
BIOMATERIALS AND BIOFLUIDS CHARACTERISATION FACILITIES
Uniaxial Testing Machine
 For uniaxial testing we use an Instron® 9543 electromechanical universal testing machine (Instron, Norwood, MA, USA) equipped with a load cell of 1 kN and a range of pneumatic and manual gripping systems to perform tensile, compression, bend, peel, tear, friction, and shear testing. Tests can be performed under quasi-static loading with continuous, static, pulsating or alternating load sequences via an electro-mechanical drive. Our machine is equipped with a temperature-controlled bath which allows performing tests in biofluid equivalent solutions at body temperature.
For uniaxial testing we use an Instron® 9543 electromechanical universal testing machine (Instron, Norwood, MA, USA) equipped with a load cell of 1 kN and a range of pneumatic and manual gripping systems to perform tensile, compression, bend, peel, tear, friction, and shear testing. Tests can be performed under quasi-static loading with continuous, static, pulsating or alternating load sequences via an electro-mechanical drive. Our machine is equipped with a temperature-controlled bath which allows performing tests in biofluid equivalent solutions at body temperature.Radial Force System
A RX675 head (Machine Solution Inc., Flagstaff, USA) is available in our platform for the characterisation of valves, stent and stent graft. The Instron 5943, equipped with this head, allows to measure the hoop force that the device exert on the anatomical portion. The head is equipped with a temperature chamber that allows to perform test at body temperature.

Biaxial Testing Machine
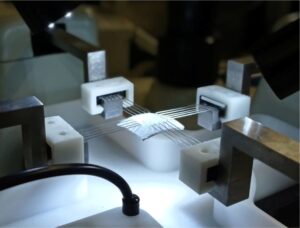 Biaxial mechanical testing are performed using a BioTester – Biaxial Tester (Cellscale, Waterloo, Canada), and allow a more accurate characterisation of soft tissues and polymeric membranes operating under multi-axial stress states. We have a range of anchoring systems, including rakes, pulleys and clamps, and a temperature controlled media bath. Displacements and strains are determined in full field by means of Digital Image Correlation technique. The system allows to perform multi-modal cyclic, simple, and relaxation testing of soft tissues and polymeric membranes over a wide range of strain rates.
Biaxial mechanical testing are performed using a BioTester – Biaxial Tester (Cellscale, Waterloo, Canada), and allow a more accurate characterisation of soft tissues and polymeric membranes operating under multi-axial stress states. We have a range of anchoring systems, including rakes, pulleys and clamps, and a temperature controlled media bath. Displacements and strains are determined in full field by means of Digital Image Correlation technique. The system allows to perform multi-modal cyclic, simple, and relaxation testing of soft tissues and polymeric membranes over a wide range of strain rates.Thermal Camera
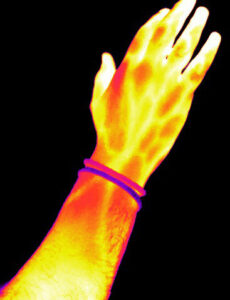 A FLIR A700 Science Kits thermal camera (Teledyne FLIR, Wilsonville, Oregon, USA) is used to perform thermoelastic stress analysis of test specimens. This is a non-destructive testing technique that allows to measure the stress distribution in a component by measuring the temperature changes it experiences when subjected to a load. In fact, a relationship exists between the temperature change and the stress-strain variation, described by the thermoelastic law.
A FLIR A700 Science Kits thermal camera (Teledyne FLIR, Wilsonville, Oregon, USA) is used to perform thermoelastic stress analysis of test specimens. This is a non-destructive testing technique that allows to measure the stress distribution in a component by measuring the temperature changes it experiences when subjected to a load. In fact, a relationship exists between the temperature change and the stress-strain variation, described by the thermoelastic law.In the case of common superelastic/shape memory alloys used in prosthetic devices, such as nitinol, the approach also allows to analyse the elasto-caloric effects introduced by phase transformation, and provide a useful tool to enhance the safety and effectiveness of critical applications based on this material.
3D Digital Image Correlation system
The system EduDIC (Dantec, Skovlunde, Denmark) is available in our platform. This system can be used for the application of 2D and 3D Digital Image Correlation, consisting of 2 cameras equipped with a 50 mm optics, a tripod, a set of targets and lights. The application of Digital Image Correlation technique to a stressed object allows to measure its surface displacement and strain, obtaining a full-field map.

Rheometer
 A Discovery Hybrid Rheometer HR10 (TA Instruments, New Castle, DE, USA) is available in our platform for fluids characterisation. This instrument measures the relationship between the applied stress and the measured deformation of a material, allowing the analysis of non-Newtonian behaviors, thixotropy, and yield stress of complex biofluids (such as blood and blood equivalents). Three different types of tests can be performed: flow, oscillatory and transient.Flow tests are used to measure the ratio between the shear stress and the shear rate, to investigate the rheological behaviour of the fluid.Oscillatory test apply a periodic stress over time, to determine the visco-elastic parameters of the material. It is also possible to vary the test conditions (e.g. temperature, frequency) to study the dependence of the visco-elastic properties. This type of test is used to study material stability and cross-linking, material properties with solvent evaporation and structure reconstruction.Transient tests are used to evaluate the behavior of the sample when subjected to constant stress/deformation, therefore the ability of the sample to fit under specific conditions.
A Discovery Hybrid Rheometer HR10 (TA Instruments, New Castle, DE, USA) is available in our platform for fluids characterisation. This instrument measures the relationship between the applied stress and the measured deformation of a material, allowing the analysis of non-Newtonian behaviors, thixotropy, and yield stress of complex biofluids (such as blood and blood equivalents). Three different types of tests can be performed: flow, oscillatory and transient.Flow tests are used to measure the ratio between the shear stress and the shear rate, to investigate the rheological behaviour of the fluid.Oscillatory test apply a periodic stress over time, to determine the visco-elastic parameters of the material. It is also possible to vary the test conditions (e.g. temperature, frequency) to study the dependence of the visco-elastic properties. This type of test is used to study material stability and cross-linking, material properties with solvent evaporation and structure reconstruction.Transient tests are used to evaluate the behavior of the sample when subjected to constant stress/deformation, therefore the ability of the sample to fit under specific conditions.PROTOTYPING FACILITIES
 We make extensive use of rapid prototyping facilities to build new testing tools, customise our equipment, create anatomical and patient specific phantoms and manufacture medical device prototypes. Our Facilities include fused deposition modelling (FDM) and Stereolithography (SLA) 3D printers.
We make extensive use of rapid prototyping facilities to build new testing tools, customise our equipment, create anatomical and patient specific phantoms and manufacture medical device prototypes. Our Facilities include fused deposition modelling (FDM) and Stereolithography (SLA) 3D printers.For FDM printing we have available a Delta WASP 2040 Pro, Wasp, Massa Lombarda RA, Italy, which allows to print with high precision (± 50 µm) large objects made from a variety of materials, including PLA, ABS, PETG, and nylon.
For SLA printing we use a Form 3+, Formlabs, Somerville, MA, USA, allowing a resolution of 25 µm and a range of general purpose materials; engineering materials with enhanced strength, chemical resistance, or heat resistance; biocompatible materials suitable for printing prosthetics and implants; and silicone.
We also have extensive experience in the use of casting and dip-molding of polymeric materials, for the creation of functional components and anatomical phantoms.
In our platform we have available a 50 watt CO2 laser cutting machine (Maitech Hobby Line CO2), designed for processing a range of non-metallic materials, including acrylic, polyurethane, leather and fabric.
The platform includes a high quality laboratory oven, the Carbolite LHT, designed for a variety of applications, which allows a heating rate of up to 15 °C/min with temperature uniformity of ±2 °C and a maximum temperature of 3000°C, allowing the thermal processing of a number of alloys.
For the manufacturing of bioprosthetic and fabric based medical devices, such as tissue valves and grafts, we use a Brother VR Embroidery Machine. This is a single-needle computer-controlled sewing and embroidery machine that precisely extract suture lines from CAD files.