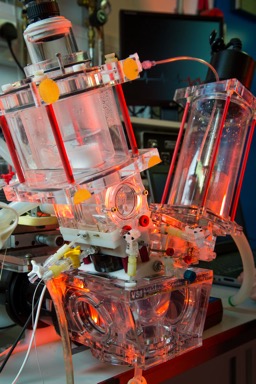
IN VITRO CARDIOVASCULAR HYDRODYNAMIC TESTING SYSTEM
To assess the performance of prosthetic heart valves under simulated cardiac conditions, we have two ViVitro Pulse Duplicator (ViVitro Labs, Inc., Victoria, BC, Canada).
This is designed to meet ISO 5840, ISO 5840-3, ISO-17845, and FDA requirements, and is capable of testing a range of prosthetic heart valves including stented tissue, percutaneous, stentless, mechanical, and TAVI/TAVR.
The system simulates physiological or other complex pressure variations of the heart function, providing detailed quality data for heart valve and system performance.
Our customised apparatus includes compliant aortic chambers with native mock leaflets for percutaneous implants assessment, micro-tip pressure transducers, aortic and mitral electromagnetic flowmeters, physiological temperature control, ultrasound viscometer for continuous monitoring of the blood equivalence of the testing fluid, additional access ports for transcatheter implantation and high speed cameras for analysis of the valve dynamics. The system is controlled with a software developed in house, allowing additional flexibility, and is coupled with PIV facilities for the visualisation and measurement of the flow parameters.

ACCELERATED DURABILITY TESTING SYSTEM
To predict the durability of heart valves, we use the VDT-3600i Heart Valve Accelerated Wear Tester (Biomedical Device Consultants and Laboratories, CO, USA). This provides temperature control and continual monitoring of the real-time differential pressures in all test stations to verify the adherence to ISO 5840 testing requirements. Also, it enables individual station alarm capabilities to halt the system in case any sample exceeds its loading requirements.
Our system, which includes up to 12 testing sections, is specifically customised to host compliant mock arteries and assess stented tissue, stentless, mechanical, percutaneous and transcatheter valves.

