Tissue Engineering Heart Valve (TEHV)
Brief description
Main topic: TEHV, development of engineered tissue and valve prostheses for the heart valve repair and replacement.
Specific objectives:
– To characterize and duplicate human heart valve structure and mechanics;
– To design, prototype and validate innovative valve prostheses with the ability to:
– Induce endogenous tissue growth;
– Increase resistance to calcification;
– Reduce thrombogenicity.
– To develop technologies and strategies for minimally invasive trans-catheter delivery approach.
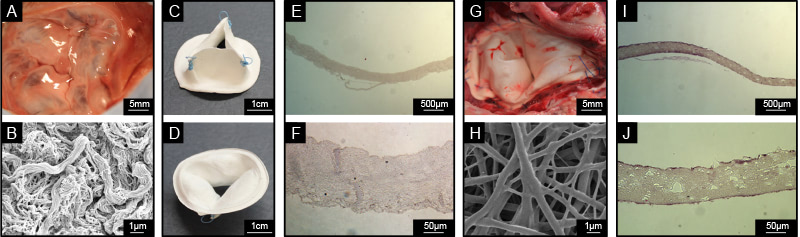
The method utilized is based on a novel polymer processing technique developed by Dr D’Amore’s group, which is named double component deposition (DCD). DCD allows for the fabrication of fibrous valve prostheses able to induce in-situ tissue growth. The fabrication method has also the ability to control micro/macro structure and mechanical properties of the engineered construct.
Impact:
Nearly 80000 patients/year require a life-saving, valve replacement in the US only. Current clinical practice for valve replacement involves two different classes of devices: mechanical valve prostheses and bio-prostheses. The mechanical valve have good longevity but require chronic anticoagulation therapy, which is in turn associated to a number of risk factors and affects the patients’ quality of life. The second category does not require chronic anticoagulation therapy and yet suffers a number of failure mechanisms with calcific degeneration being one of the most frequent. Technologies developed by Dr D’Amore’ s team aim to overcome the limitations of these two classes of medical devices by introducing engineered heart valves able to re-adjust to somatic growth, resist to calcification and do not require anticoagulants. This research line is functional to develop advanced polymer processing techniques, which can be utilized for different applications. Last, these research efforts are also focusing the prototyping of novel hybrid medical devices based on combined biodegradable metallic and polymeric components.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic area:
Products:
Medical devices & tissue engineering
Collaborations:
- University of Pittsburgh, Pittsburgh, Stati Uniti
- UPMC, Pittsburgh, Stati Uniti
- University of Cincinnati, Cincinnati, Stati Uniti
- IRCCS ISMETT, Palermo, Italia
- West Virginia University, Morgantown, Stati Uniti
- Harvard Medical School, Boston, Stati Uniti
- Universidade Estadual de Campinas, Campinas, Brasile
- University of Texas at Austin, Austin, Stati Uniti
Scarica il pdf del progetto
