Tolerogenic Dendritic Cells therapy for early weaning of Liver Transplanted patients
Abstract
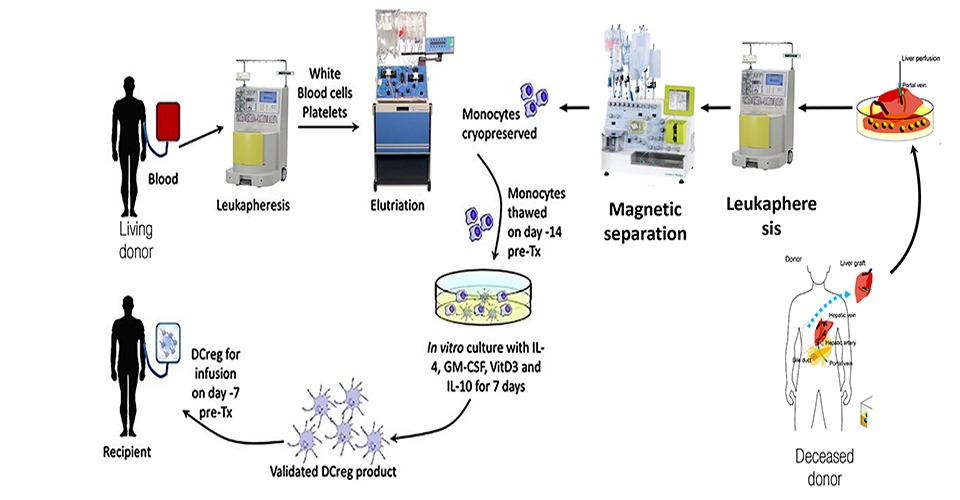
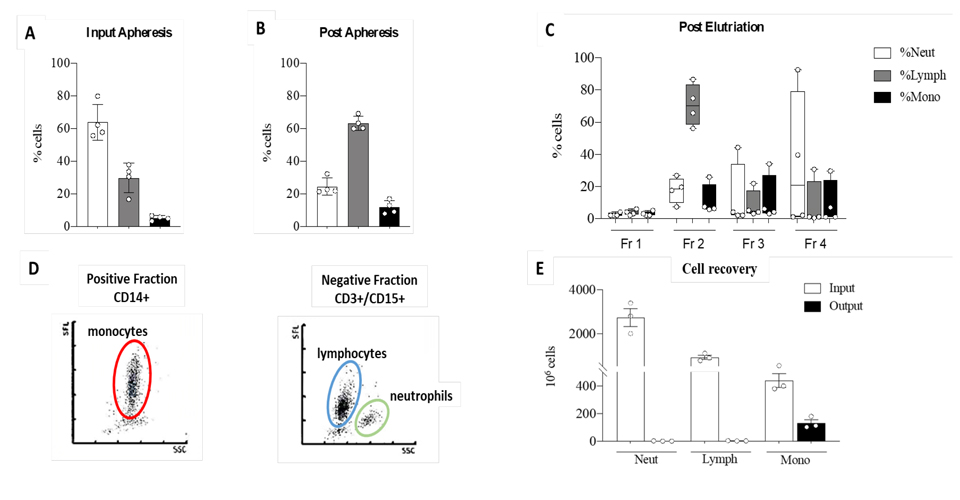
One major caveat of organ transplantation is the occurrence of an unwanted immune response to the graft. Accordingly, immunosuppressive therapy is provided for life to transplanted patients, though it is accompanied by severe side effects such as kidney failure and others. It is therefore important to explore alternative curative methods based, for instance, on the use of cellular therapies based on the administration of donor derived tolerogenic Dendritic Cells for early weaning of liver transplanted patients. The aim of this study is the optimization of a cell mediated therapy to promote operational immune tolerance in liver recipients using tolerogenic Dendritic Cells (DCreg) obtained from the liver perfusate of deceased-donors. During liver procurement from brain-dead-heart-beating donors, the aorta is clamped and liver perfused though the hepatic vein. The liver perfusate thus obtained is routinely discarded. However, liver perfusate contains an incredible amount of blood borne cells circulating to/from tissues, among which DCs precursors. These CD14+ monocytes are cultured for 7 days with IL4/GM-CSF/IL-10 and Vitamin-D3 and examined in vitro for their immunosuppressive potential against alloreactive donor-derived T cells.
Impact:
Patients who undergo solid organ transplant require lifelong immunosuppression to prevent organ rejection. Immunosuppressive therapy are associated with life-threatening side effects such as infection, malignancy, diabetes, cardiovascular disease and renal failure. In organ transplantation, the ideal form of immunosuppression is to induce donor specific tolerance without impairing the host defenses or increasing the susceptibility to infection from all types of organisms. Dendritic Cells, if opportunely redirected, can serve to induce long term tolerance to donor alloantigen by inducing donor-specific T cell hypo-responsiveness and memory to donor alloantigen. DCreg functionally prevent organ rejection and early weaning from immunosuppressive therapy in transplanted patients. The Phase I/II protocol optimized by our collaborators in Pittsburgh (Prof AW Thomson) consists in the use of tolerogenic Dendritic cells obtained from the peripheral blood of liver living donors.
The frequency of living donors transplants is drastically lower than deceased donors. The possibility to use DCreg for the early weaning of immunosuppressive therapy in the cohort of patients that receive livers from decease donors would increase the number of treatable individuals using infusion of DCreg. The choice of liver transplantation is promising as the liver is an immune-tolerant organ per se. to date, no alternative therapies for the induction of operational immune tolerance have been proposed. The therapy proposed in this study could increase the number of retained transplants and could be applied also to other solid organs different than liver such as kidneys.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
ATMP (Advanced Therapy Medicinal Products)
Collaborations:
- Thomas E. Starzl Transplantation Institute, University of Pittsburgh, USA
Scarica il pdf del progetto