Development of an engineered hyperelastic scaffold for tendon enthesis regeneration
Brief description
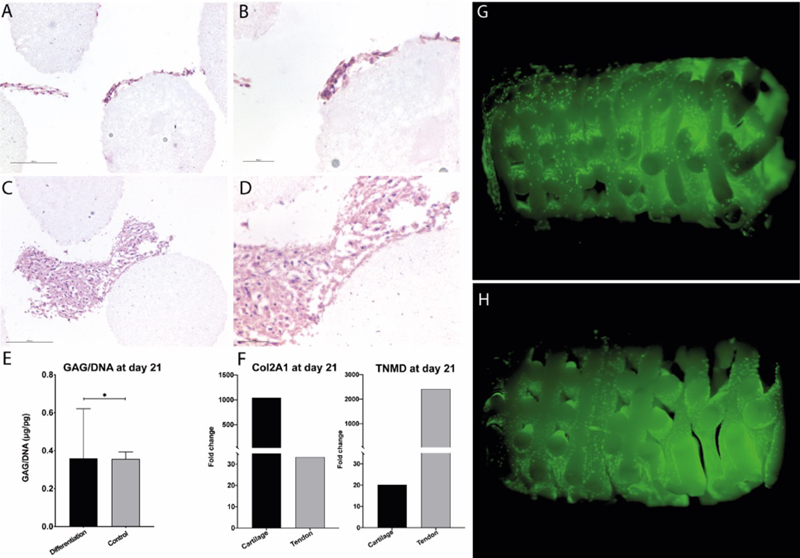
The tendon-to-bone enthesis (TTBE) is a specialized connective tissue structure essential to guarantee a smooth transition between tendons and bones. Injuries affecting the TTBE have high clinical incidence especially in the elderly and in the more active populations that play sports, at both the professional and non-professional level. It is estimated that each year in the EU and the USA about 30 million people undergo tendon/ligament repair procedures, causing an annual expense of over €150 billion. Despite several innovative techniques that have been developed, surgical repair of massive enthesis injuries is still inadequate, with up to 79% failure rate in the most severe cases. In this project we fabricated a cellularized graft mimicking the tendon-fibrocartilaginous biphasic transition tissue of the TTBE. The construct is composed of a tendon-like side and a cartilage-like side, cellularized with adult human mesenchymal stem cells (hMSCs) The structural core of our construct is a scaffold composed of medical grade poly(L-lactide-co-glycolide) (PLGA), with high internal porosity and elastic properties sufficient to form a self-supporting mesh, and able to support cell proliferation.
Impact:
With this project we aimed to develop a construct mimicking the tendon-to-bone enthesis to satisfy the clinical need of high-performance constructs to be used in reconstructive tendons surgery. Our engineered construct promoted the differentiation of stem cells toward cartilaginous and tendinous phenotypes. To achieve this we used an innovative, biphasic bioreactor that is part of the Intellectual Property portfolio of Ri.MED Foundation and that we have previously employed to engineer other biphasic tissues. In terms of materials, the high tensile properties of hyperelastic PLGA scaffold confer mechanical resistance to our construct. Hence, our construct may support the surgical repair of tendons injuries, promoting a fast healing and improving the post-surgery outcomes.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic area:
Products:
Medical devices & tissue engineering
Collaborations:
University of Pennsylvania (UPenn), United States
Bioengineering and Biomaterials Laboratory, Children’s Hospital of Philadelphia (CHOP), United States
Center for Cellular and Molecular Engineering (CCME), University of Pittsburgh, United States
Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, United States
Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, United States
The Chinese University of Hong Kong, China
Cell Biology Inspired Tissue Engineering, Institute for Technology-Inspired Regenerative Medicine, Maastricht University, Netherlands
MERLN Institute for Technology-Inspired Regenerative Medicine, Maastricht University, Netherlands
Scarica il pdf del progetto
