Novel regenerative platforms to grow surrogate organs using stem/progenitor cells
Abstract
Irreversible organ failure remains a major global challenge due to the shortage of transplantable organs. Although many tissues have been engineered in vitro, successful clinical translation has largely been limited to thin or low-metabolic tissues, as vascularizing larger organs remains difficult. To address this, we developed an in vivo vascularized tissue-engineering model by implanting target cells or tissues into lymph nodes (LNs). LNs rapidly expand to accommodate new cells and possess immediate vascular access, making them ideal bioreactors for growing functional organs.
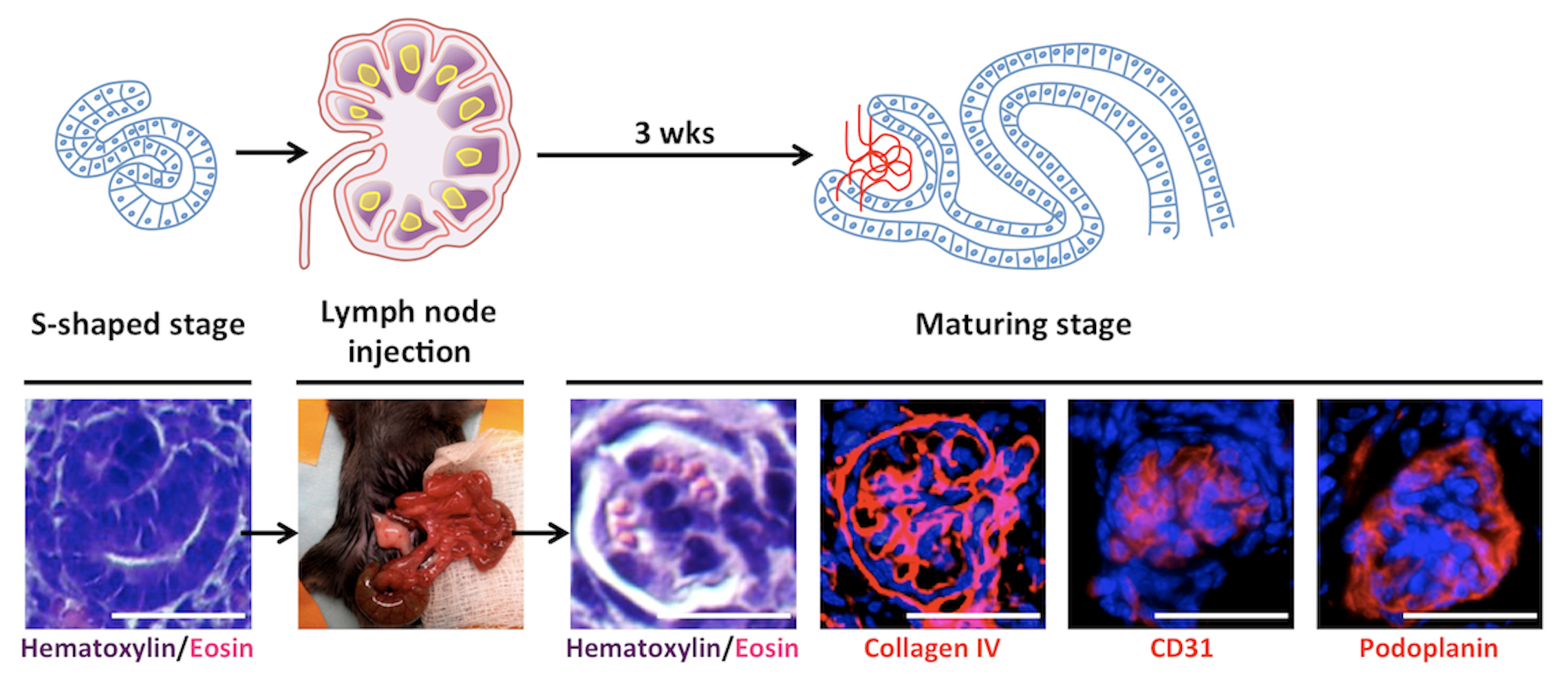
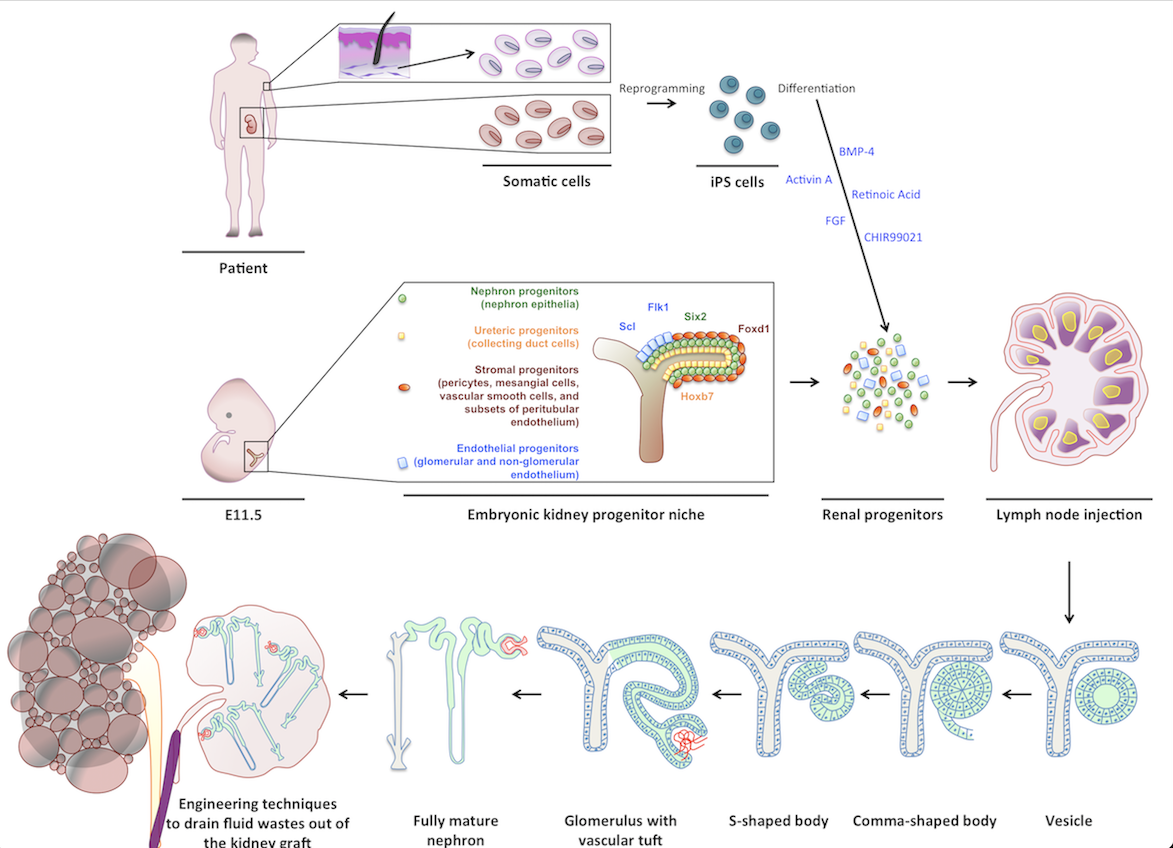
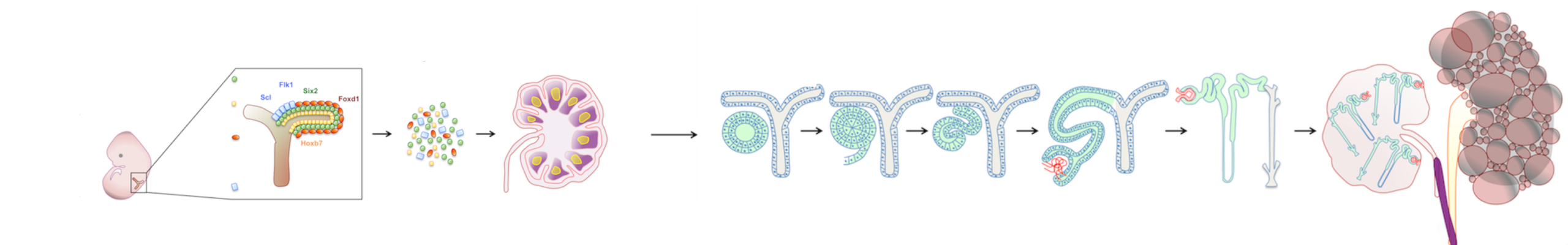
My early work examined whether LNs could support maturation of various embryonic tissues, eventually focusing on kidney regeneration. Kidneys are the most in-demand organ for transplantation, yet current approaches such as recellularizing decellularized scaffolds have not yielded mature, functioning renal tissue. In contrast, both mouse and human kidney rudiments implanted into LNs matured into structures with excretory, homeostatic, and endocrine functions. The LN also supported engraftment and maturation of mouse nephron progenitors and human iPSC-derived renal cells. Mechanistic studies showed that lymphoid stromal Lymphotoxin beta receptor signaling is essential for angiogenesis of transplanted renal tissues in LNs and in the omentum.
We found that fat-associated lymphoid clusters (FALCs) also support organogenesis, enabling the formation of functional ectopic livers capable of rescuing tyrosinemic animals.
These studies have led to two patents describing LNs and FALCs as sites for organogenesis and to the founding of LyGenesis Inc., a company developing LN-based regenerative therapies. During my volunteer work with the company, I assisted in isolating GMP-grade hepatocytes as LyGenesis progressed toward clinical translation.
LyGenesis received FDA clearance in 2020 for a Phase 2a trial using allogeneic hepatocyte transplantation into periduodenal LNs for end-stage liver disease (NCT04496479). Following initial dosing, the DSMB approved dose escalation in March 2025.
In addition to its kidney platform, which remains at the preclinical in vivo stage, the company is developing an ectopic thymus platform, also at the preclinical stage, in which I actively participated. My unpublished work demonstrated dose-dependent T-cell production in athymic mice following LN-based thymus transplantation, sex-specific differences in T-cell regeneration, and rejuvenation of T-cell function in aged females. This work resulted in an additional patent on thymic tissue transplantation.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contatto
Aree terapeutiche:
Prodotto:
Organi artificiali
Collaborazioni:
University of Pittsburgh, Department of Pathology. Pittsburgh, Pennsylvania, USA.
McGowan Institute for Regenerative Medicine. Pittsburgh, Pennsylvania, USA.
Mayo Clinic. Rochester, Minnesota, USA
Saint Barnabas Medical Center. Livingston, New Jersey, USA
Scarica il pdf del progetto