Molecular mechanisms of protein misfolding diseases
Brief description
Neurodegenerative diseases constitute a heterogeneous family of disorders, each characterized by the loss of neurons in a specific region of the nervous system and resulting in specific clinical symptoms. However, they all seem to share the same molecular mechanism involving the progressive accumulation of protein aggregates in nervous tissue, that cause toxicity and subsequent cell death.
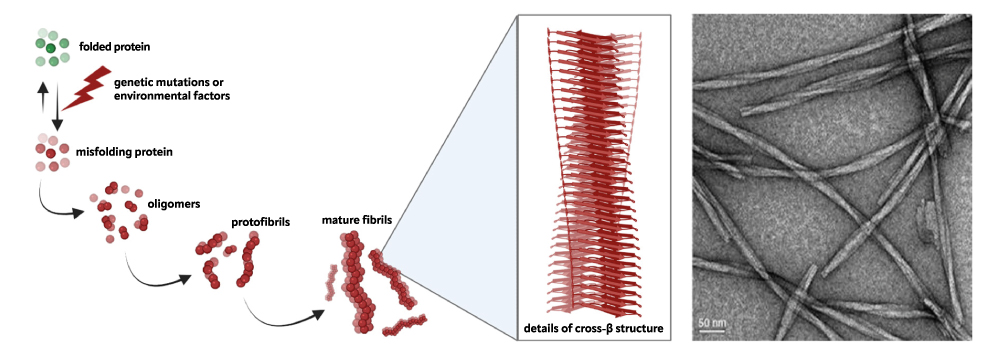
Specifically, a mutation or a stochastic event can destabilize a particular protein, unique for each neurodegenerative condition. This protein loses its proper folding and self-organizes into insoluble amyloid structures, against which the cell’s normal control systems prove ineffective. A detailed study of this pathological mechanism is, therefore, crucial to explore potential therapeutic strategies aimed at slowing down or, ideally, inhibiting the formation of these protein aggregates.
In this context, our research is focused on understanding the molecular mechanisms leading to protein aggregation and characterizing specific protein-ligand interactions. These interactions can help us in designing anti-aggregating molecules capable of targeting a specific site, limiting side effects. Our current work is aimed at studying the pathological aggregation mechanisms of Ataxin-3 and TDP-43 proteins, which are involved in Spinocerebellar Ataxia Type 3 (SCA3) and Amyotrophic Lateral Sclerosis (ALS), respectively.
We use a biophysical approach, involving the use of Nuclear Magnetic Resonance spectroscopy, Fluorescence spectroscopy, calorimetry, and interferometry techniques. Furthermore, we have access to gold standard Structural Biology facilities for Small Angle X rays Scattering (SAXS), for low resolution, and X-ray crystallography for atomic resolution. We also employ advanced microscopy techniques (e.g. Transmission Electron Microscopy and Atomic Force Microscopy) for morphological studies of post-treatment protein aggregates.
Impact
The progress made in biomedicine have extended our lifespans. Nevertheless, this extended longevity has brought with it a sharp rise in the occurrence of debilitating health conditions as people age. This has made neurodegenerative diseases a daily burden for millions of suffering patients and their families, as well as a large sanitary emergency for governments. Unfortunately, current treatments are, in the best case, palliative and poorly effective, reflecting a limited understanding of the molecular process of most of these diseases.
Lack of a deep understanding of the molecular mechanisms of neurodegenerative diseases leads to inefficient and non-specific therapies. Our research deepens into both structural and cellular biology, revealing critical insights to neurodegenerative diseases mechanisms. The knowledge gained will assist in the design of specific therapies and, at the same time, provide important clues regarding the fundamental phenomenon of protein folding and assembly. Biophysical studies, along with structural analyses, are crucial for better defining the molecular basis of the pathological mechanism of these highly aggregation-prone proteins under more manageable conditions, using in vitro assays with pure recombinant proteins. The information obtained can be used both for the design of more specific and effective anti-aggregating compounds capable of interfering with the pathological supramolecular assembly, and for validating innovative in vitro models that can mimic the complexity of in vivo systems.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
Drugs – Biologics
Collaborations:
- UK Dementia Research Institute (UK DRI) – King’s College London, Londra, Regno Unito
- Dipartimento di Fisica e Chimica (DiFC) – Università degli Studi di Palermo, Palermo, Italia
- Institute of Nanotechnology (CNR Nanotec) – CNR, Lecce, Italia
- i3S Institute for Research and Innovation in Health, Porto, Portugal
