CAR-NK cells engineering for hepatocellular carcinoma cell therapy
Abstract
HCC is a malignant epithelial tumor arising from hepatocytes and is often associated with chronic hepatitis and cirrhosis caused by hepatitis B or hepatitis C virus infections. Treatments for HCC include hepatectomy, liver transplant or chemotherapy are not effective for advanced forms of HCC and the risk of recurrence is high. Therefore novel treatment strategies are needed to improve the prognosis of HCC.
Immunotherapy is one such therapy that functions differently from conventional treatments. Natural killer (NK) cells play an important role in the innate host immune response against viruses and tumors. The frequency and function of NK cells in the peripheral blood and liver are associated with recurrence and survival rates of patients with resectable HCC. Thus, hepatic NK cells are thought to play an important role in mediating the immune function of the liver and immunological defense mechanisms against HCC.
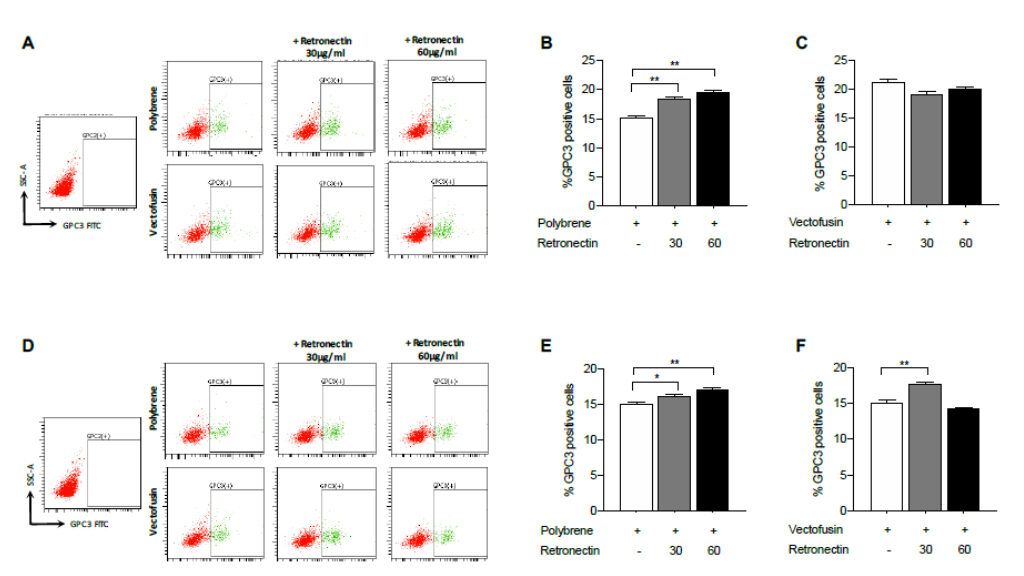
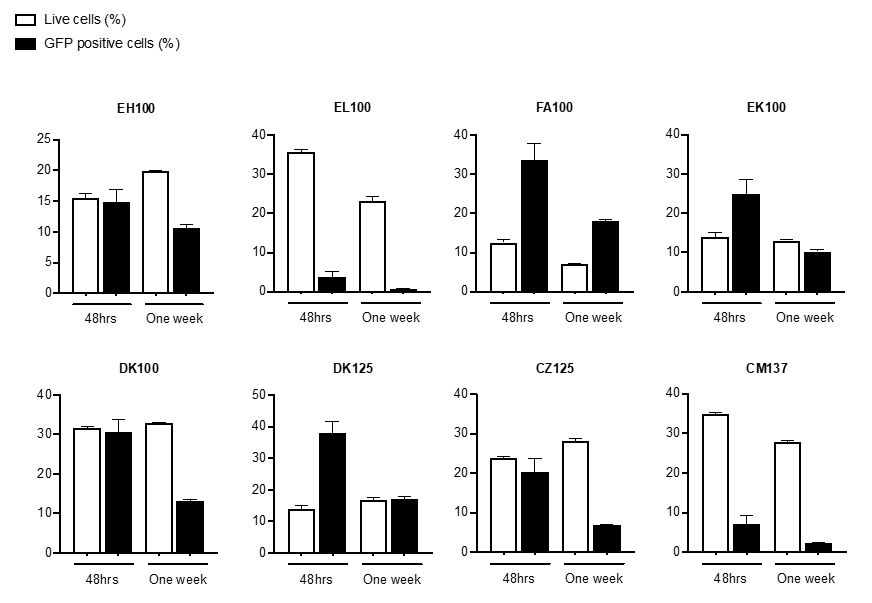
Genetic modification techniques have been developed to improve the specificity and efficacy of NK cell cytotoxicity to tumor cells. For example, the approach using CAR for NK cells has improved the specificity and efficacy of NK cell therapy. In this study, we propose the use of a novel CAR construct that combines the induced tumor antigen specificity of a Tumor Associated Antigen (TAA) described in HCC into NK cell.
In addition, NK cells will be engineered to produce IFN that our preliminary data have proven to significantly enhance NK cells response to tumor and infection. The vector we designed is a fourth generation construct harboring the signaling domain specific for TAA antigen followed by a transmembrane signaling domain, CD28 and 4-1BB co-stimulatory intracytoplasmic domains and the suicide gene Epidermal Growth Factor Receptor in a truncated form (EGFRt).
The truncated form is inert as it has lost its function and does not respond to the growth factor, thus avoiding unwanted and uncontrolled responses. Furthermore, the receptor specifically binds to the antibody Cetuximab and in vivo binding to EGFRt leads to death of target cells by complement fixation.
Impact:
CAR-engineering has found more ground in T cell-mediated therapies. However, CAR-NK cells have several advantages over CAR-T cells. CAR-NK cells reportedly reduce the risks of autoimmune response and neoplastic transformation because they have a shorter lifetime than CAR-T cells. In addition, cytokines released from NK cells, such as IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF), are considered safer than the cytokine storm that results from CAR-T cell therapy.
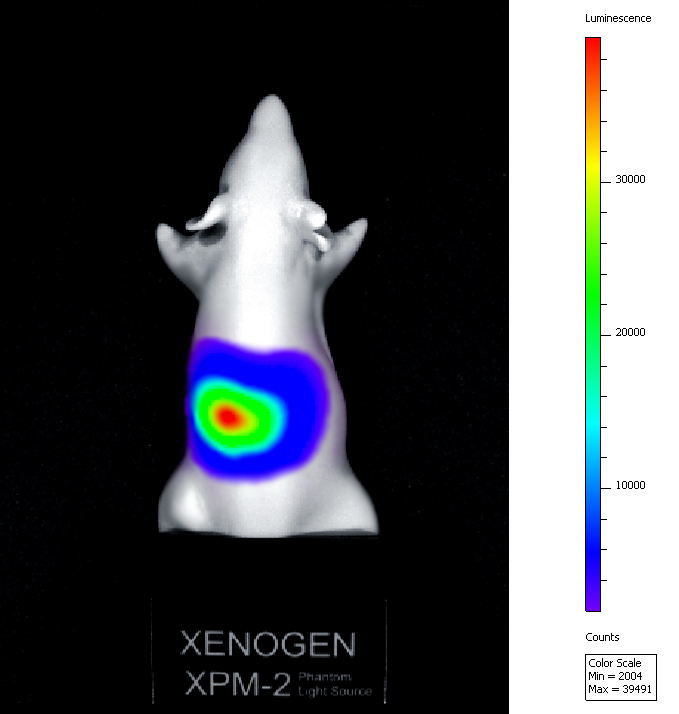
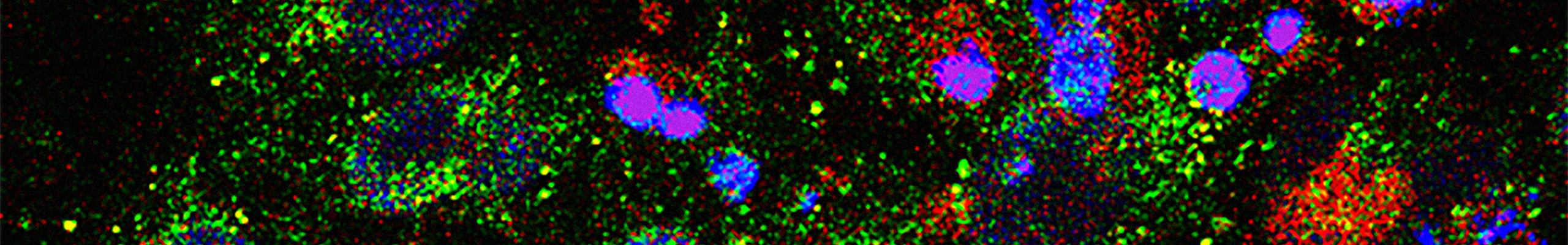
Unlike CAR-T cells, CAR-NK cells retain an intrinsic capacity to recognize and target tumour cells through their native receptors, making the escaping of tumour cells through downregulation of the CAR target antigen less likely. Lastly, NK cells do not require strict HLA matching and lack the potential to cause graft-versus-host disease, an important risk imposed by CAR-T cell immunotherapy, which make it possible for CAR-NK cells to be an “off-the-shelf” allogeneic therapeutic option. In our study, we will genetically engineer human primary NK cells with a CAR protein specific for a Liver Tumor Associated Antigen. Our goal is to obtain CAR-NK cells using a GMP-compliant scalable virus-free method of gene editing.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
ATMP (Advanced Therapy Medicinal Products
Collaborations:
- Istituto Zooprofilattico Sicilia IZS, Palermo, Italia
- Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione (IRCCS ISMETT), Palermo, Italia
Scarica il pdf del progetto