Advancing cholangiocyte organoids for regenerative medicine and tissue engineering
Abstract
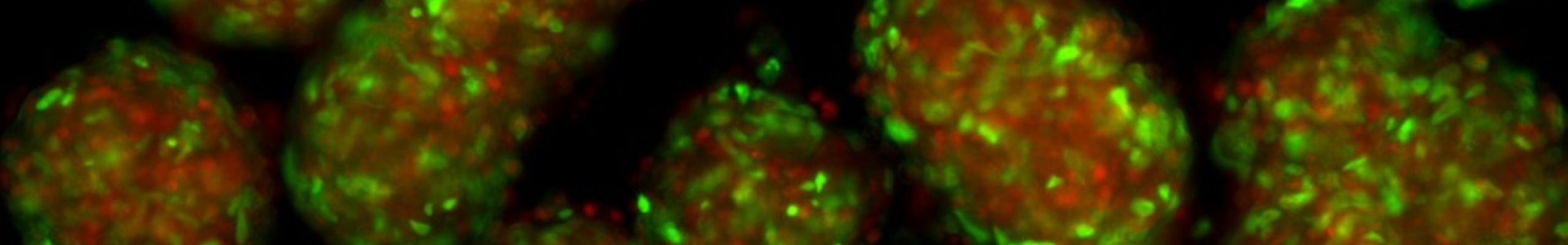
Three-dimensional (3D) organoid systems offer significant advantages over traditional two-dimensional (2D) cultures but are limited by their reliance on variable, animal-derived matrices such as Matrigel. Matrix-free approaches can overcome this limitation but often struggle to achieve proper tissue organization—a challenge that can be addressed by incorporating supportive cells such as mesenchymal stromal cells (MSCs), which provide structural extracellular matrix to support tissue architecture, as well as pro-angiogenic cues that guide vascular integration of organoids in vivo.
Building on this principle, we developed a simple, efficient, and cost-effective method to generate matrix-free co-culture organoids of cholangiocytes and MSCs using orbital shaking. These co-culture organoids exhibited enhanced cholangiocyte maturation, developed endothelial-like cells, and demonstrated greater regenerative potential than cholangiocyte monocultures, as shown by successful repopulation of decellularized biliary scaffolds.
Consistent reproducibility and functional reliability are critical for advancing organoid research. To establish robust quality control, we measured the size, weight, and mass density of individual organoids using the W8 Physical Cytometer, assessing intra- and inter-batch variability as well as structural differences between monocultures and co-cultures. These analyses revealed that MSCs contribute significantly to heterogeneity, likely reflecting their diverse behaviors in the 3D microenvironment, including ECM deposition, differentiation, and paracrine signaling.
Within the CASTOR&POLLUX project (Center for Acquisition, STOrage, and Processing of Multi-Omics Data from Three-Dimensional Cellular Models; D3 4 Health Initiative – Digital Driven Diagnostics, Prognostics and Therapeutics for Sustainable Health Care, Spoke 4), the molecular profiles of organoids will be analyzed to identify key pathways, providing an additional level of control that will not only allow the selection of reproducible and clinically relevant organoids but also guide their development through optimized culture protocols.
Impact:
Our integrated approach, combining biophysical and molecular features, advances cholangiocyte organoid technology toward reproducible, physiologically relevant, and clinically translatable models, offering a versatile platform for disease modeling, regenerative medicine, and tissue engineering.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contatto
Team di progetto:
Ayesha Rehman, PhD
Giovanni Cavalli
Aree terapeutiche
Prodotto
Biomarcatori – Organi artificiali
Collaborazioni
ISMETT – Istituto Mediterraneo per i Trapianti Ismett IRCCS
Scarica il pdf del progetto
