Development and characterization of a bioengineered bile duct
Abstract
This research program aims to restore biliary function by creating tissue-engineered implants for different segments of the biliary tree, starting with the choledochus (CBD)—the distal segment of the extrahepatic bile duct connecting the liver to the duodenum. The clinical need for an artificial CBD arises from a spectrum of cholangiopathies—including biliary atresia, distal cholangiocarcinoma, choledochal cysts, and particularly iatrogenic injuries following liver transplantation or other upper-abdominal procedures—that often lead to progressive strictures, inflammation, and ultimately liver failure. Conventional treatments for strictures, such as plastic stents, require frequent replacement, while self-expanding metal stents—despite longer patency—carry risks of migration and tissue ingrowth. Biodegradable scaffolds offer a promising alternative due to their natural in vivo degradation; however, despite research efforts, no bioengineered biodegradable bile duct has reached the clinic. This is largely because it remains challenging to align scaffold degradation with tissue regeneration, ensure appropriate biocompatibility and mechanical properties, and select suitable cellular sources.
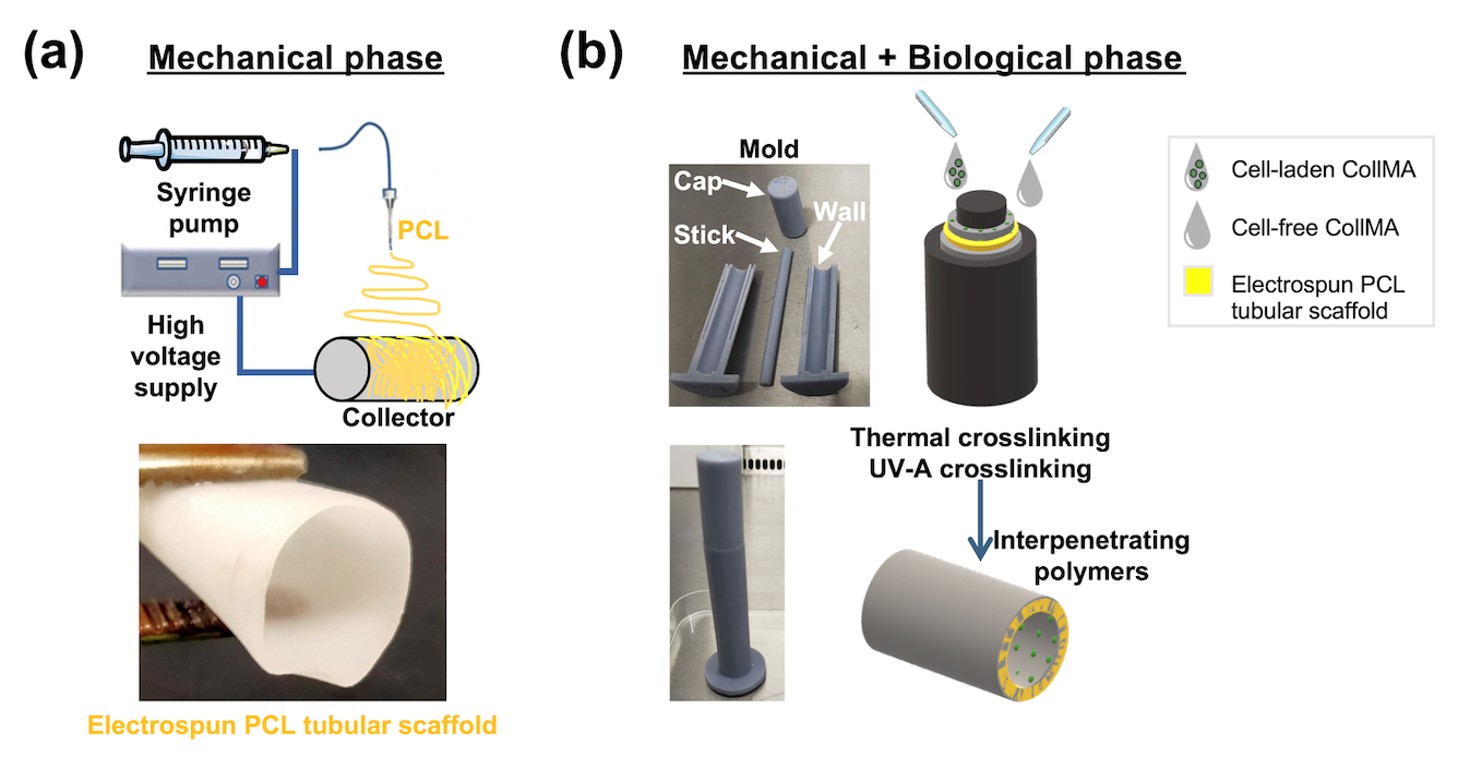
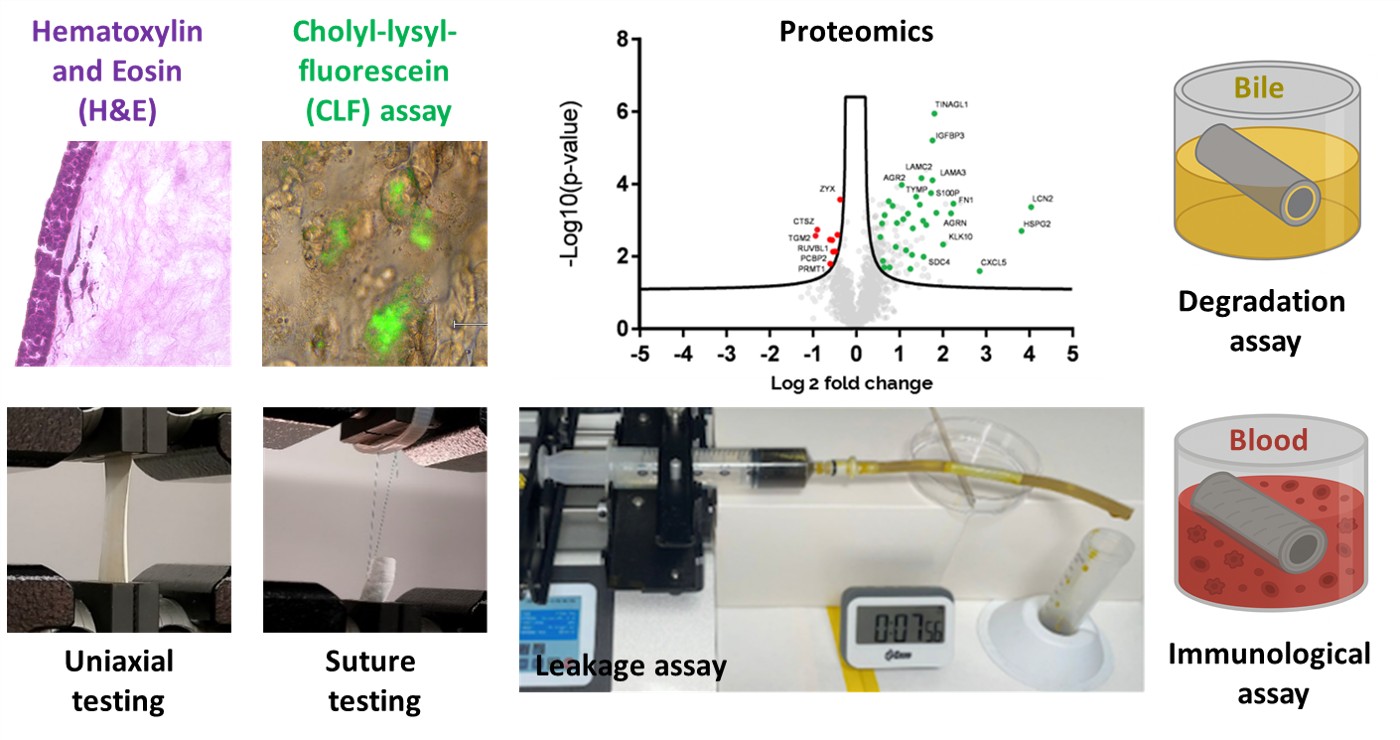
To address this unmet need, we developed a novel multiphasic tubular scaffold that closely mimics the size and function of the human CBD. Composed of two biocompatible biomaterials and seeded with biliary epithelial cells, the construct demonstrates excellent homogeneity, stability, mechanical strength, suturability, and leak-proof performance. Its ability to transport and modify bile acids highlights functional maturity and suitability for in vivo applications. Initial ex vivo human blood assays of the acellular scaffold revealed a favorable immune profile, with limited inflammation and sustained epidermal growth factor release, supporting its regenerative potential. This innovation—currently protected through a patent application—represents a significant advance in bioengineered bile duct repair.
Ongoing work focuses on enhancing the biliary epithelial component and incorporating additional cell types to improve physiological relevance. In particular, we are developing self-organizing biliary organoids composed of epithelial, mesenchymal stromal, and endothelial cells as an improved cellular source for the scaffold.
Impact:
The development of a bioartificial CBD represents a transformative advance in treating cholangiopathies that necessitate replacement of this segment. By integrating biodegradable biomaterials with a biologically active cellular component and rigorously evaluating the construct in preclinical models, our approach addresses critical limitations of previous strategies. This work not only provides a pathway toward clinical translation but also establishes a versatile platform for engineering other segments of the biliary tree, potentially offering long-term solutions for patients with complex biliary diseases.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Team di progetto:
Therapeutic Areas:
Product:
ATMP (Advanced Therapy Medicinal Products) – Dispositivi biomedicali e Organi artificiali
Collaborations:
Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione (IRCCS ISMETT), Palermo, Italia
Scarica il pdf del progetto